5-Methyl-MDA is mentioned for the first time by Shulgin & Nichols1 and does look like a pretty interesting compound. It substitutes for (+)-MBDB, MMAI, and DOI in their rat models suggesting effects something like perhaps a combination of MDMA and LSD. It has less than one-tenth the potency of MDA as a dopamine uptake inhibitor, which would prevent the serototoxic effects associated with MDA due to extracellular dopamine. It is more than 5 times more potent than MDA in inhibiting uptake of serotonin suggesting a suitable human dosage of 15-25 mg. It would be interesting to hear reports of human consumption of this novel substance. An open question - Is the dopamine uptake inhibition of MDA and MDMA an essential component of their empathogenic properties? The experimental is enclosed here and it seems pretty straight-forward. 2-Methyl-MDA has about the same pharmacological profile as 5-Methyl-MDA, but 6-Methyl-MDA is much less potent, even less so than MDA.
Experimental
5-Dimethylaminomethyl-4-hydroxy-3-methoxybenzaldehyde2
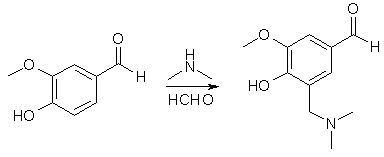
To a stirred solution of 37% aqueous CH2O (120 g, 1.5 mol) and 40% aqueous dimethylamine (180 g, 1.5 mol) in 900 mL of EtOH was added vanillin (152 g, 1 mol). The mixture was refluxed for 30 min, stirred at 25°C for 24 h and then refrigerated overnight. The white granular solid was collected by filtration, washed with ice-cold acetone and then dried under vacuum to give 179.8 g (86%) of 5-Dimethylaminomethyl-4-hydroxy- 3-methoxy-benzaldehyde, mp 139-141°C.
4-Hydroxy-3-methoxy-5-methylbenzaldehyde2
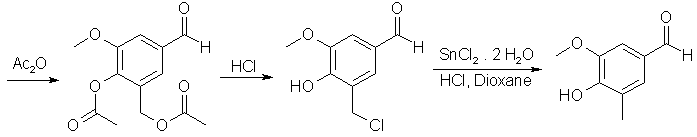
A solution of the Mannich base 5-Dimethylaminomethyl-4-hydroxy-3-methoxybenzaldehyde (104.5g, 500 mmol), in 500 mL of Ac2O was refluxed for 24 h protected from moisture. Volatile materials were removed by distillation (bp 55-80°C) under aspirator vacuum. After cooling the residue (which was the crude diacetate) to ~40°C, 500 mL of conc. HCl was added gradually and then the mixture was stirred at ambient temperature for 1.5 h by which time most of the 5-chloromethyl derivative had precipitated. Enough p-dioxane (~400 mL) was added to ensure dissolution of the solid at 60-70°C. Then with efficient (mechanical) stirring 337.5 g (1.5 mol) of SnCl2·2H2O was added and the mixture was refluxed for 30 min. After cooling to 25°C and dilution with 200 mL conc. HCl, the mixture was extracted with CHCl3 (5x150 mL). The combined organic layers were washed with 6 N HCl, water, 70% NaCl solution and then evaporated in vacuo to dryness. The crude residue was adsorbed on silica gel (63-200 mesh, 70 g) by adding silica gel to a solution of the crude in CHCl3 and then evaporating the solvent in vacuo. A slurry of the mixture in CHCl3 was then applied on a column of silica gel, (100 g) and eluted with ether-hexane (2:1). Evaporation of eluents followed by sublimation (0.1 mm, 120°C) of the grey solid residue gave 70.6 g (85%) of 4-Hydroxy-3-methoxy-5-methylbenzaldehyde as a white solid: mp 99-101°C.
3,4-dihydroxy-5-methylbenzaldehyde3,4
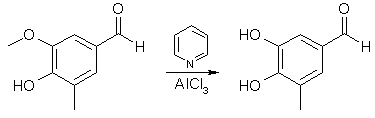
Anhydrous AlCl3 (32.4 g, 0.242 mol) was suspended in a solution of 36.6 g (0.220 mol) of 4-hydroxy-5-methoxy-3-methyl- benzaldehyde in 300 mL of CH2Cl2 in an apparatus protected from atmospheric moisture. While stirring briskly and cooling to maintain the temperature at 30-35°C, 76.6 g (0.970 mol) of pyridine was added slowly. The reaction was vigorous; the solution of the reaction complex was heated to reflux (45°C) and maintained at that temperature with stirring for 24 hours. The solution, which had darkened only slightly during the reflux period, was cooled to 25°C and the product was hydrolyzed, while stirring and maintaining the temperature at 25-30°C, by the addition of dilute (15-20%) HCl until the mixture was definitely acidic to congo red indicator. Extraction of the aqueous phase with ether followed by evaporation of the ether left 25.4 g (76 %) of 4,5-dihydroxy-3-methylbenzaldehyde. An analytical sample was prepared from recrystallization from EtOAc-cyclohexane: mp 186-188°C.
5-Methyl-piperonal1,5
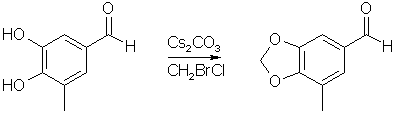
To a mechanically stirred suspension of 3,4-dihydroxy-5-methyl- benzaldehyde (12.84g, 84.5 mmol) and cesium carbonate (41.28g, 126.7 mmol) in 200ml anhydrous DMF was added bromochloromethane (8.23ml = 16.39g, 126.7 mmol), and the resulting mixture heated to 110°C for 2h. The reaction mixture was then cooled to room temperature and filtered through a pad of celite and the filter cake washed with ethyl acetate. The filtrate and washings was concentrated almost to dryness, diluted with water and extracted three times with ethyl acetate. The extracts were washed with water and brine, dried over MgSO4, filtered and evaporated to give 13.54g (98%) of a tan solid, sufficiently pure for the next step. An analytical sample was obtained by kugelrohr distillation of 1.27g to give 1.05g of a white solid, mp 45-46°C, bp 120°C/0.25 mmHg.
3,4-Methylenedioxy-5-methylphenyl-2-nitropropene1
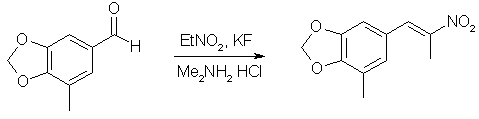
A mixture of 11g (67.1 mmol) 5-Methyl-piperonal, 40ml nitroethane, 10.9g (134 mmol) of dimethylamine hydrochloride, 0.58g (10 mmol) potassium fluoride and 40 ml toluene was placed in a flask equipped with a dean-stark trap and refluxed for 24h under nitrogen. The solvents were evaporated under vacuum, and the residue partitioned between ether and water. The etheral layer was dried over MgSO4 and evaporated to give 14.12g (95%) of product as an orange-yellow solid. An analytical sample was recrystallized from methanol to give pale orange crystals, mp 97-98°C.
5-Methyl-MDA1

A solution of 2.21g (10 mmol) 3,4-Methylenedioxy- 5-methylphenyl-2-nitro- propene in 20 ml THF was added dropwise to a suspension of 2.65g (70 mmol) LiAlH4in 25ml THF while stirring under nitrogen. The reaction mixture was stirred and heated under reflux for 5 h, then cooled to room temp and the excess hydride quenched carefully by the sequential addition of 2 ml IPA, 2ml 15% NaOH and 7ml water. The precipitate was removed by filtration, and the resulting solution was evaporated. The residue was suspended in water and acidified with conc HCl, washed three times with ether, made basic with 25% NaOH and extracted three times with ether. The ether was dried over MgSO4, evaporated and the residue distilled under vacuum. The distilled oil was dissolved in 10ml IPA, neutralized with ethanolic HCl and diluted with ether to precipitate 1.26g (65% yield) 5-Methyl-MDA hydrochloride, mp 214-215°C.