Abstract
Methyleugenol 1a was oxidized to give 3,4-dimethoxyphenylacetaldehyde by the oxidative system containing the RuCl3-NaIO4-phase transfer catalyst. The yield and spectroscopic properties were obtained from the stable acetaldoxim 3a. Furthermore, this oxidation system could be applied to other arylpropenes, thus, safrole, 4-methoxyallylbenzene, allylbenzene, and the corresponding arylacetaldehyde formed.
Arylacetaldehyde is used for perfume compositions1 or as a useful intermediate of drugs, especially, 3,4-dimethoxyphenylacetaldehyde is an important intermediate of verapamil, a cardiac drug2.
The synthetic methods for preparing the arylacetaldehydes by oxidation from the corresponding allyl compounds using ozone3,4, OsO4/NaIO45–9 or HCO2H-30%H2O2/Pb(OAc)410–12 have been reported. In addition, a number of preparations for arylacetaldehyde have been known, such as:
- Rearrangement of styrene oxides13
- Decarboxylation of phenylglycidate14
- Synthesis via enamine of α-oxophenylpropanoic acids15
On the other hand, there are many synthetic methods using the oxidative cleavage of alkenes with a catalytic amount of ruthenium metal16, however few results have been reported on the oxidative cleavage of allyl compounds to produce the corresponding arylacetaldehydes.
Recently, we have reported the safe and facile oxidation of β-pinene to produce nopinone using RuCl3-NaIO4-PTC (phase transfer catalyst) system17. As an extension of this oxidation system, we now report the synthesis of 3,4-dimethoxyphenylacetaldehyde 2a from methyleugenol 1a.
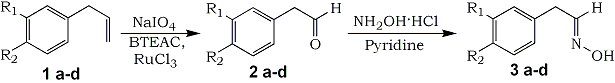
We have found that the oxidation of methyleugenol 1a using 1 mol% of RuCl3, 5 mol% of benzyltriethylammonium chloride (BTEAC), and 5 mol equiv. of NaIO4 in EtOAc/H2O for 2 h at room temperature gave 3,4-dimethoxy-phenylacetaldehyde 2a with 100% conversion and 95% selectivity by GC. The other 5% by-product was found to be 3,4-dimethoxybenzaldehyde by GC-MS (M+=166). The oxidation conditions, i.e., the oxidant, solvent, and amount of catalyst were nearly fixed according to our previous report17 (see experimental section). We also applied this oxidative system to other 3-aryl-1-propenes such as safrole 1b, 4-methoxyallylbenzene 1c, and allylbenzene 1d, and thus the corresponding arylacetaldehydes 2b–d were obtained. Since the peroxidation and/or polymerization of 2, the yield could not be shown as optimized values. Therefore, the yield and spectroscopic properties were obtained after transformation to the arylacetaldoxims 3a–d which are useful precursors of amines or nitriles (Table 1). This oxidation did not proceed in the absence of RuCl3 as a catalyst. We also have investigated the effect of PTC. All of the PTCs smoothly promoted the oxidation to give good conversion and selectivity of 2a in comparison with the GC analysis value obtained in the absence of PTC as shown in Table 2.
Table 1.
Physical data of arylacetaldoxim 3.
| 3 | R1 | R2 | Yielda | Melting Pointb (Lit. mp) |
| a | MeO | MeO | 65% | 54–55°C (90°C7) |
| b | -OCH2O- | 57% | 116–117°C (115°C3) | |
| c | H | MeO | 63% | 58–63°C (120°C18) |
| d | H | H | 66% | 66–68°C (98°C19)c |
a. Yield is based on 1.
b. M.p. of mixture of syn:anti isomers.
c. M.p. of reference is a syn isomer.
Table 2.
The effect of PTC in the oxidation of 1a.
Runa |
PTC | Product 2a |
|
Convb |
Selectb |
||
1 |
No addition | 49% |
90% |
2 |
n-Bu4NI | 100% |
93% |
3 |
n-Bu4NClO4 | 100% |
92% |
4 |
BnEt3NCl | 100% |
95% |
5 |
(n-Oct)3MeNCl | 92% |
94% |
6 |
n-Bu4PBr | 95% |
87% |
7 |
BnPh3PCl | 90% |
85% |
a. Reaction conditions: 1, 45 mmol;
RuCl3, 0.25 mmol; PTC, 2.2 mmol;
NaIO4, 225 mmol; EtOAc/H2O, r.t., 2 h.
b. Conversion yield and selectivity
of 2a were determined by GC.
In conclusion, we performed the syntheses of arylacetaldehydes from 3-aryl-1-propenes using RuCl3-NaIO4-PTC system.
Experimental
All reagents and solvents were obtained from commercial sources and used without further purification. Melting points: Yanagimoto micromelting apparatus, uncorrected values.
Typical Procedure: 3,4-Dimetoxyphenylacetaldehyde (2a) and Aldoxim (3a)
To a solution of methyleugenol 1a (8.0 g, 45 mmol), RuCl3 (50 mg, 0.25 mmol), and benzyltriethylammonium chloride (0.5 g, 2.2 mmol) in EtOAc (80 mL), NaIO4 (47.5 g, 225 mmol) in water (500 mL) was added slowly for 1 h at room temperature. The resulting solution was stirred for additional 1 h. EtOAc (250 mL) was added to the reaction mixture. The organic layer was separated, washed with water, dried with anhydrous MgSO4 and concentrated in vacuo to give an oil (6.3 g, 74%). The purity was determined to be 95% by GC. To the crude product, hydroxylamine hydrochloride (7.8 g, 112 mmol), pyridine (6 mL), and EtOH (44 mL) were added and refluxed for 1 h. After removal of solvent in vacuo, EtOAc (50 mL) and water (50 mL) were added, the organic layer was washed with 5% HCl, brine, dried with anhydrous MgSO4, and concentrated in vacuo to give 3a (5.7 g, 65% based on 1a, anti:syn = 1:1; by 1H NMR) as a solid.