Abstract
The acylative decarboxylation reaction of phenylacetic acid, a method used in clandestine laboratories for the synthesis of phenyl-2-propanone, has been examined. It has been demonstrated that this reaction is accompanied by side-reactions that produce characteristic neutral compounds. some of these are cis- and trans-1,3-diphenyl-2-methylpropene and the enol acetates derived from dibenzylketone and phenyl-2-propanone. It would be difficult for an unskilled chemist to separate these neutral substances from phenyl-2-propanone, therefore illicit phenyl-2-propanone is likely to be an impure product. The use of crude phenyl-2-propanone in reductive amination reactions aimed at amphetamine production gives rise to a complex mixture of amines and characteristic neutral compounds. The likely origins of N-acetylamphetamine and amphetamine benzaldimine are also discussed.
Amphetamine and methylamphetamine are common illicit drugs in Australia. Importation of these drugs appears to be a rare phenomenon and this indicates that most of the material is synthesized in local clandestine laboratories. Most laboratories appear to be engaged in either the reduction of ephedrine using hydriodic acid and red phosphorus, or the reductive amination of phenyl-2-propanone, often by the Leuckart route1,2.
Chemical supply companies and the police are aware of the potential use of phenyl-2-propanone and therefore illicit drug chemists are denied ready access to this starting material. It is therefore likely that most clandestine laboratories engaged in reductive amination reactions in Australia now use homemade phenyl-2-propanone.
There were two goals for the work described herein: one was to identify two neutral substances found in product confiscated from an illicit laboratory engaged in the synthesis of amphetamine; and the other was to explore the origin of these substances, which appeared to be related to the use of illicit phenyl-2-propanone in a Leuckart reaction.
Discussion
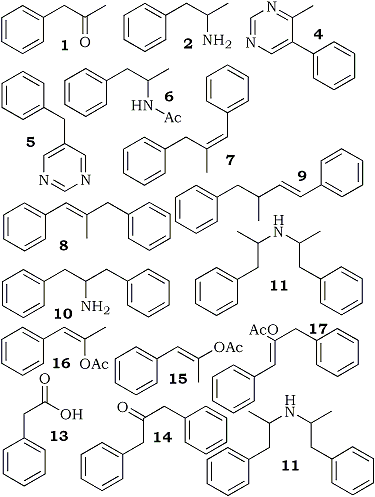
Fig 2.
Chemical structures of substances found in Mixture A.
Figure 1 shows a gas chromatogram produced from an oily, deep brown liquid seized from an illicit laboratory while Fig. 2 shows the chemical structures of substances found within the liquid; this liquid will be referred to as Mixture A.
In addition to amphetamine (peak 2 in Fig. 1, structure 2 in Fig, 2) and dodecane (peak 3, internal standard). 1,3-diphenyl-2-aminopropane (peak 10, structure 10), di-(1-phenyl-2-propyl)amine (two peaks, one for each diastereoisomer, at 11 , structure 11), N-formyl di-(1-phenyl-2-propyl)amine (two peaks, one for each diastereoisomer, at 12, structure 12) N-acetylamphetamine (peak 6, structure 6), 4-methyl-5-phenylpyrimidine (peak 4, structure 4). 4-benzylpyrimidine (peak 5, structure 5), and amphetamine benzaldimine (peak 9, structure 9, stereochemistry uncertain) were found. This combination of substances indicates that the liquid was the product from a reductive amination of phenyl-2-propanone1,2, probably under Leuckart conditions3-5.
Gas chromatography-mass spectrometry (GC-MS) showed that peaks 7 and 8 were not known impurities produced by the Leuckart reaction, nor any other commonly used amphetamine synthesis. The fragmentation patterns of the two substances were virtually identical and allowed them to be tentatively identified as cis and trans isomers of 1,3-diphenyl-2-methylpropene (7) and (8). This identification was confirmed by synthesizing the two alkenes unambiguously using a modification of the method of Bumgardner6.
As these alkenes are not likely by-products in reductive amination reactions it was postulated that they arose during the synthesis of the putative starting material, phenyl-2-propanone, and were carried through ensuing synthetic steps. The presence of 1,3-diphenyl-2-aminopropane (10) in the illicit mixture strongly suggested that dibenzylketone (14) was present in the starting material used in the reductive amination7-9; this in turn suggested that the starting material had been prepared using acylative decarboxylation of phenylacetic acid (13)7-9. According to Frank10 sodium acetate and acetic anhydride are commonly used to accomplish this transformation. Fig. 3 depicts the likely mechanism by which the reaction proceeds.
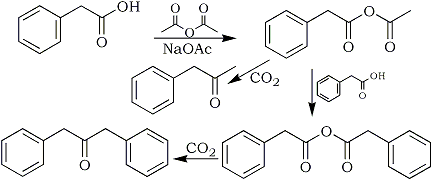
Fig. 3
The likely mechanism by which the reaction proceeds.
Under the conditions shown in Fig. 3 it is possible that the alkenes (7) and (8) could arise from a decarboxylative Knoevenagel-type condensation between phenylacetic acid and the newly formed phenyl-2-propanone (Fig. 4).
In order to test this postulate the acylative decarboxylation of phenylacetic acid was examined, and phenyl-2-propanone was treated with phenylacetic acid in the presence of sodium acetate. The strategy used during these experiments was to conduct the reactions under conditions that a "backyard" chemist might use. that is to say, not under an anhydrous or oxygen free atmosphere.
Fig. 5 is the gas-liquid chromatogram produced by the product of the acylative decarboxylation after extraction from sodium hydroxide (see Experimental section: this mixture of substances will be referred to as Mixture B), Peaks 1, 3, 13, and 14 are due to phenyl-2-propanone, dodecane (internal standard), phenylacetic acid and dibenzylketone, respectively, with peaks 7 and 8 due to cis and trans 1,3-diphenyl-2-methylpropene. The postulated source of the alkenes in Mixture A was therefore confirmed.
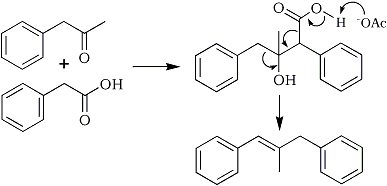
Fig. 4
The mechanism that likely produced the two alkenes.
When a solution of sodium acetate and phenylacetic acid in phenyl-2-propanone was heated at about 100°C for 22 h traces of the two alkenes were produced. This observation adds weight to our postulate that the two alkenes are produced by the mechanism depicted in Fig. 4.
Unexpectedly, Mixture B was also found to contain three other substances in relatively high abundance (peaks 15, 16 and 17). Preparative liquid chromatography allowed (15) and (16) to be separated from the mixture. Mass spectrometry and nuclear magnetic resonance spectroscopy suggested that these two substances were the cis and trans enol acetates of phenyl-2-propanone. The mass spectral fragmentation pattern for peak 17 suggested that this substance was the enol acetate of dibenzylketone (17); the stereochemistry of this substance was assumed to be trans. Work directed towards the unambiguous synthesis of these three enol acetates and the confirmation of their presence in Mixture B is continuing in our laboratory. If these enol acetates are produced as byproducts in the synthesis of phenyl-2-propanone it is very unlikely that the crude purification processes used by illicit chemists would be capable of separating them, or the alkenes (7) and (8), from the desired product. Therefore illicit reductive amination reactions probably make use of phenyl-2-propanone contaminated with these substances. This might explain why illicit amphetamine mixtures are sometimes found to contain N-acetylamphetamine (6); the enol acetates might acylate amphetamine as it is formed. We are currently examining this possibility.
Throughout the course of many reactions that we have conducted using phenyl-2-propanone it has become apparent that it tends to decompose to benzaldehyde. From our tests it is not yet clear whether heat, or oxygen, or both are responsible for this decomposition. In any event, the conditions used for reductive amination of phenyl-2-propanone are conducive to the formation of benzaldehyde; this would account for the presence of amphetamine benzaldimine (9) in illicit amination products.
Experimental
Synthesis of Cis and Trans 1,3-Diphenyl-2-Methylpropene
1,3-Diphenyl-2-methyl-2-propanol was prepared by treating dibenzylketone with methylmagnesium iodide using the conditions of Bumgardner6. The alcohol thus produced was dehydrated by distillation from chromatographic grade alumina at elevated temperature (Bunsen flame) and reduced pressure (ca 1 mm Hg). A mixture of three alkenes, identified as cis and trans 1,3-diphenyl-2-methylpropene (7) and (8) and 2-methylene-1,3-diphenylpropane on the basis of their nuclear magnetic resonance and mass spectral data6, was collected as distillate.
GC-MS of the mixture was performed using a 15 m x 220 µm x 0.25 µm DB1 capillary column coupled to a JEOL DMX 303 spectrometer operating in the electron impact mode at 70 eV. Mass spectral data are presented in the Appendix.
Acylative Decarboxylation of Phenylacetic Acid
A mixture of phenylacetic acid (5 g, 35 mM), sodium acetate (2.5 g, 30 mM) and acetic anhydride (7.3 mL, 80 mM) was heated at reflux for 22 h. After the mixture had been allowed to cool, it was treated with water (2.5 mL) and basified to pH 9 with aqueous sodium hydroxide solution (25%, approximately 14 mL). This solution was extracted with diethyl ether (3 x 20 mL) and the combined organic extracts dried over anhydrous sodium sulfate.
Gas chromatography of the dried extract (Mixture B) was performed on a 15 m x 220 µm x 0.25 µm DB1 fused silica capillary column using H2 as carrier gas with U = 55 cm/sec and flame ionization detection. The oven was kept at 100°C for 2.5 min then programmed to 270°C at a rate of 30°C/min. The gas chromatograms of Mixture A and Mixture B were obtained using these conditions (see Figs. 1 and 5).
A small aliquot of the ethereal extract was concentrated under a stream of nitrogen and then chromatographed on silica gel (BDH 60-120 mesh) using light petroleum as the mobile phase to yield two fractions. One was rich in cis and trans 1,3-diphenyl-2-methylpropene (7) and (8), while the other was rich in substances tentatively identified as the cis and trans isomers of 2-acetoxy-1-phenyl-1-propene (15) and (16). For the latter mixture the following proton magnetic resonance data was collected in CDCl3 at 300 MHz: δ 2.12 and 2.16 (2 x S, 3H), 2.23 (S, 3H), 6.08 and 6.37 (2 x S, 1H), 7.25 to 7.66 (complex, 5H).
Mass spectral data were obtained for compounds (15), (16), and (17) using conditions identical to those described above; see Appendix for spectra.
Conclusion
It has been demonstrated that the acylative decarboxylation of phenylacetic acid is accompanied by side reactions. In the hands of an unskilled chemist the substances produced by these reactions would be difficult, if not impossible, to separate from the desired product, phenyl-2-propanone. The use of crude phenyl-2-propanone in reductive aminations gives rise to a complex mixture of amines and characteristic neutral compounds.
Appendix
Top Spectrum
Mass spectrum due to peak 17 from Fig. 5; tentatively identified as the trans enol acetate of dibenzyl ketone (17). See Experimental section for details.
Middle Spectrum
Mass spectrum due to peak 16 from Fig. 5; tentatively identified as the conjugated cis enol acetate of phenyl-2-propanone (16). The trans isomer (15) has a mass spectrum that is virtually identical.
Bottom Spectrum
Mass spectrum due to peak 8 in Fig. 1; identified as trans-1,3-diphenyl-2-methylpropene (8). The cis isomer (7) has a mass spectrum that is virtually identical.