Aryl iodides have been used as pivotal precursors in organic synthesis and prepared by various reactions with iodine or other iodine compounds (Kl, ICI, IF, N-iodoamides).1 However, most of the reactions require the presence of Lewis acid, base or oxidizer to produce a reactive iodine species2 Also, drastic conditions such as a strong acidic medium or a strong oxidizer in stoichiometric amounts have been used in many cases for the effective iodination of an aromatic ring.3
In this paper, we would like to report a new and convenient synthetic method for the iodination of an aromatic ring using I2 and nitrate. To a solution of anisole (3 mmol) and I2 (1.7 mmol) in acetic acid (10 mL) was added sodium nitrate (0.3 mmol) at room temperature. The reaction mixture in a condenser-attached flask was heated at 85°C for 6 hrs under a deoxygenated argon atmosphere. During the reaction, three additional portions of sodium nitrate (3x0.3 mmol) were added respectively at the first every hour. The resulting mixture was treated with 10% aqueous NaHSO3 solution (20 mL) and extracted with ether (3x30 mL). The organic extracts were washed with brine (20 mL) and dried with Na2SO4. Evaporation of solvents in vacuo gave the crude iodination product. The crude product was purified by flash chromatography (hexane/benzene) with silica gel to afford 4-iodoanisole in 92% yield.
By this new method, various aromatic rings with an electron-donating group can be successfully iodinated in good yield in the absence of strong acid (Table 1). It is quite peculiar that neither a strong acid nor acetic anhydride is necessary for the iodination. And the reaction needs only cheap and readily available chemicals. Moreover, the directional selectivity of iodination is excellent to produce para-iodoaromatic ring except for toluene.
A change of the counter-cations of nitrate (NaNO3, KNO3 or Ca(NO3)2) makes little effect on the result of iodination. Thus, it is apparent that I2 is activated by the action of NO3- not by any metallic cations.
The novel iodination with I2/NO3- performed under a deoxygenated argon atmosphere reveals that 0.4 equivalent of nitrate is the least amount required for the quantitative iodination of anisole (Figure 1). On the other hand, 0.2 equivalent or less of nitrate is enough for the complete iodination if oxygen is bubbled through the reaction mixture. These results can be explained by the following equation5 which shows the formation of 3 equivalents of reactive I+ based on the quantity of nitrate used.
NO3- + 4 H+ + 1.5 I2
3 I+ + NO + 2 H2O
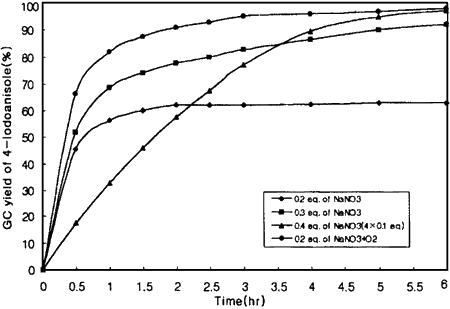
Figure 1.
Effect of NO3- quantity on the iodination of anisole at 85°C.
In the presence of oxygen, however, a tentative intermediate NO might be further oxidized to NO+ followed by the oxidation of another I2 to I+.3a
The fact that weakly-activated substrates (toluene, xylene) necessitate an elevated reaction temperature and more quantity of nitrate than 0.4 equivalent for a satisfactory iodination might be attributed to a relatively low reactivity of the substrates with I+ and partial loss5 of NO, oxidizing species at high temperature. In contrast, N-substituted carbazoles, strongly activated aromatic compounds, seem to undergo different reaction pathway since the iodination requires only 0.1 equivalent of nitrate in the absence of oxygen.
It is also interesting to note that the treatment of I2/NO3- on phenylacetylene produces 1-iodo-2-nitro-1-phenylethene as a major product and no iodinated aromatic ring.6 This result sheds an important clue for the understanding of iodination mechanism7 and demonstrates the existence of INO2 species8 during a reaction with I2/NO3-.
In summary, an easy and novel synthetic method using I2/NO3- is developed for the effective iodination of aromatic ring with an electron-donating group. This method does not require any strong acid or oxygen. In addition, under an oxygen atmosphere only 0.2 equivalent of nitrate is enough for the successful iodination.
| Substrate | Nitrate | Nitrate (mmol) |
Temp. |
Time |
Yieldb |
Productc |
| anisole | NaNO3 | 1.2 | 85°C |
6 h |
92% | 4-iodoanisole |
| anisole | NaNO3 | 0.6 | 85°C |
4 h |
92%d | 4-iodoanisole |
| anisole | KNO3 | 1.2 | 85°C |
6 h |
90% | 4-iodoanisole |
| anisole | Ca(NO3)2·4 H2O | 0.6 | 85°C |
6 h |
92% | 4-iodoanisole |
| anisole | — | 0 | 85°C |
6 h |
- | 4-iodoanisole |
| diphenylether | NaNO3 | 2.4 | 85°C |
21 h |
70%e | 4,4'-diiodo-diphenylether |
| 9-methylcarbazole | NaNO3 | 0.6 | 30°C |
21 h |
87%e | 3,6-diiodo- 9-methylcarbazole |
| 9-benzylcarbazole | NaNO3 | 0.6 | 30°C |
10 h |
88%e |
9-benzyl-3,6-diiodocarbazole4 |
| aniline | Ca(NO3)2·4 H2O | 0.6 | 65°C |
30 h |
37% | 4-iodoacetanilide |
| acetanilide | Ca(NO3)2·4 H2O | 0.6 | 105°C |
24 h |
47%f | 4-iodoacetanilide |
| durene | NaNO3 | 1.2 | 116°C |
6 h |
65% | 1-iodo-2,3,5,6- tetramethylbenzene |
| mesitylene | NaNO3 | 1.2 | 116°C |
4 h |
81% | 1-iodo-2,4,6- trimethylbenzene |
| m-xylene | Ca(NO3)2·H2O | 1.2 | 116°C |
2 h |
90% | 1-iodo-2,4-dimethylbenzene |
| toluene | Ca(NO3)2·4 H2O | 3.0 | 116°C |
2.5 h |
61%g | iodotoluenes |
| chlorobenzene | Ca(NO3)2·4 H2O | 3.0 | 116°C |
13 h |
- | 1-chloro-4-iodobenzene |
Notes:
- 3.0 mmol of substrate, 1.7 mmol of I2, nitrate and 10 mL of
AcOH were used under argon unless otherwise specified. - Yields of pure and isolated products.
- All the structures were identified by IR, NMR data and melting points.
- Oxygen was bubbled through the reaction mixture.
- 3.3 mmol of I2 were used.
- GC yield.
- para:ortho=61:39.