Dear bees,
Last week Otto searched some journals and came across this: "A simple and Convenient Method for Epoxidation of Olefins without Metal Catalysts", from Advanced Synthesis and Catalysis 345, No.3, 389-392 (2003).
They use Bleach to epoxidize various alkenes, styrenes work best. Only drawbacks are the use of acetonitrile and a phosphate buffer.
The experimental procedure
| Alkene | Time | Yield |
|---|---|---|
| Styrene | 60 min | 80% |
| Propenylbenzene | 120 min | 87% |
| 4-MeO-Styrene | 20 min | 90% |
In a 100mL Schlenk tube, KBr (357 mg, 3.0 mmol or 47 mg, 0.4 mmol), buffer (10 mL, prepared by adjusting a 0.5 M KH2PO4 solution to a pH of 10.4 with 2M NaOH, CH3CN (10 mL), and alkene substrate (2.0 mmol) were added. The reaction mixture was warmed to 40°C under 1000 rpm magnetic stirring using a thermostat. Aqueous NaOCl solution (1.1 eq.) was added and the mixture was extracted with 20 mL of EtOAc. The combined organic layers were dried over MgSO4, the solvent evaporated in vacuo and the crude epoxide was purified by flash chromatography (Hexane/EtOAc 10:1) or distillation.
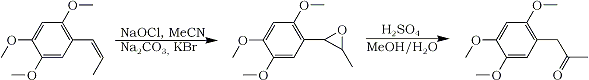
The next day otto tried it. After some trials he found, the pH of the buffer isn't that important (at least for the asarone) and it can be replaced by sodium carbonate. The needed amount of bleach (Sodium Hypochlorite) was higher than suggested by the literature, perhaps due to some impurities in the asarone.
TLC Rf values
(Hexane/EtOAc 4:1)
| Asarone | 0.4 |
| Epoxide | 0.1 |
| Diol | 0.0 |
| Ketone (TMP2P) | 0.2 |
All visible in UV or
by KMnO4-reagent.
Experimental
In a 100mL beaker 2 mmol asarone (416 mg, 385 uL; ottos asarone was vacuum distilled from calamus oil), 10 mL acetonitrile, 10 mL tap water, about 400 mg sodium carbonate1 and 350 mg potassium bromide (3 mmol)2 were mixed. Then, 1.5 equivalents of bleach3 was added and the mixture stirred for 15 to 20 minutes4. The mixture was extracted once with 15 mL Dichloromethane5 and the dried organic phase evaporated to give 360 mg (80%) fairly pure asarone epoxide6.
This epoxide was rearranged to ketone by the usual H2SO4 method. 350 mg gave only 75 mg ketone after purification. A key to why that is probably because of asarone being very acid-sensitive.
Notes
- This amount worked fine. less may work, too.
- This amount can propably be reduced to as little as 50 mg; with higher amounts yields of epoxide are higher according to the literature.
- 1.1 eq like in the lit. left unreacted asarone as confirmed by TLC.
- Do not exceed this time! the epoxide slowly hydrolyses giving the diol and other stuff.
- Any solvent capable of extracting the epoxide should do it. DCM is just easy to remove.
- As confirmed by TLC.