10% Palladium on charcoal, 3 atm H21a
As shown in Scheme 1, the nitrostyrene (3) was obtained by the reaction of 4-methoxy- 2,3-methylenedioxybenzaldehyde1b,c (2) with MeNO2. lt was reduced to the amine (4) hydrochloride by catalytic hydrogenation in a MeOH and 1N HCl mixture over 10% Pd/C catalyst with a yield of 67%. Because of the insolubility of 3 in solvents commonly used for catalytic hydrogenation, this method for the reduction of nitrostyrene (3) on a larger scale could not be applied. Alternatively, the reduction could be done with LiAlH4 in dry THF.
Scheme 1
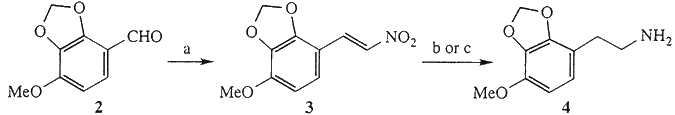
a) MeNO2, AcONH4/AcOH, 100°C. b) H2, 10% Pd/C in MeOH, aq. 1 N HCl, rt. c) LiAlH4/THF, 80°C.
Experimental
General
TLC was carried out on precoated silica gel 60 HF254 plates (Merck). Silica gel 60 (230-400 mesh, ASTM, Merck) was used for column chromatography.
4-Methoxy-2,3-methylenedioxy-β-nitrostyrene (3)1c
4-Methoxy-2,3-methylenedioxybenzaldehyde1b,c (2) (4.49 g; 24.9 mmol), MeNO2 (3.3 mL; 61 mmol), AcONH4 (1.94 g) in AcOH (20 mL) were heated at 100°C for 3 h under argon. After cooling, the reaction mixture was poured into cold H2O (150 mL). The crystalline precipitate was filtered, washed with H2O and dried to give fine yellow crystals: 4.65 g (83%); mp 156.2-159.8°C (MeOH) (lit.1c 155°C (dec.))
IR (KBr) |
1640s, 1620s, 1595, 1515s, 1500s, 1450s, 1445s, 1335s, 1290s, 1270s, 1205, 1175, 1110s; |
1H-NMR (CDCl3) |
7.87 (d, J = 13.5, 1H), 7.76 (d, J = 13.5, 1H), 6.92 (d, J =8.7, 1H), 6.60 (d, J =8.7, 1H), 6.12 (s, 2H), 3.96 (s, 3H); |
13C-NMR (CDCl3) |
148.4 (s), 146.8 (s), 137.3 (s), 135.6 (d), 134.0 (d), 125.7 (d), 108.3 (d), 107.2 (s), 102.4 (t), 56.6 (q); |
CIMS (NH3) |
241 (100, [M+NH4]+), 224 (6, [M+1]+), 223 (6, M+·) |
2-(4-Methoxy-2,3-methylenedioxyphenyl)ethylamine (4)
- Catalytic reduction
- Nitrostyrene (3) (0.5 g; 2.24 mmol) was dissolved in MeOH (150 mL). To this solution aq. 1N HCl (11 mL, 5 equiv.) and Pd/C (10%, 0.1 g) were added. This was continuously shaken overnight under a H2 atmosphere (50 psi). After filtration over Celite®, the solution was evaporated to dryness to yield a brownish yellow solid. Treating this solid with CH2Cl2 gave a colorless precipitate which was filtered and dried to give 4·HCl (0.35 g, 67%); mp 215.8-216.3°C (MeOH-Et2O).
- Reduction with LiAlH4
- LiAlH4 (1.2 g; 31 mmol) was suspended in dry THF (110 mL), and then heated to reflux under N2. To this refluxing suspension, the solution of nitrostyrene (3) (1.14 g; 5.1 mmol) in dry THF (20 mL) was added dropwise during 2 h and refluxed for 3 h under N2. After cooling, sat. aq. sodium potassium tartrate solution was added and the mixture extracted with Et2O (6x30 mL). After drying of the organic phase (Na2SO4), it was evaporated to dryness to give a dark yellow oil, which was purified by acid/base treatment: 0.7 g (72%).
IR (KBr) |
3400 (br), 3130 (br), 3050s, 1645, 1505, 1450, 1405s, 1290, 1275, 1170, 1140, 1090, 1050 |
1H-NMR (CD3OD) |
6.62 (d, J = 8.6, 1H), 6.49 (d, J = 8.6, 1H), 5.88 (s, 2H), 3.78 (s, 3H), 3.08 (t, J =7.4, 2H), 2.82 (t, J = 7.4, 2H); |
13C-NMR (DMSO-d6) |
146.9 (s), 142.9 (s), 134.9 (s), 122.9 (d), 108.2 (d), 101.4 (t), 56.6 (q), 38.7 (t), 26.9 (t); |
CIMS (NH3) |
196 (100, [M+1]+), 195 (19, M+·) |