1-Phenyl-1-chloro-2-methylaminopropane (I) was first prepared by Emde1 in a study of ephedrine derivatives, continuing earlier studies by Schmidt2 who had prepared the corresponding 1-phenyl-1-bromo-2-methylaminopropane and reduced it to desoxyephedrine (1-phenyl-2-methylaminopropane) with zinc-copper and hydrochloric acid. His yield, as reported later by his student Emde, was 10%. Emde hydrogenated both I and Schmidt's bromine compound catalytically to desoxyephedrine in yields of 80-90%.
Both authors obtained a hydrocarbon as a by-product, which Schmidt identified as propylbenzene, while Emde writes of propenylbenzene PhCH=CHCH3 without giving any proof of identity other than the smell of the hydrocarbon.
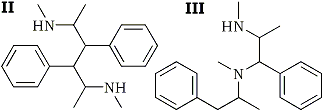
Emde also found a high-molecular base in his hydrogenation mixture. Because the analysis and molecular weight of this base corresponded approximately to a dimer of desoxyephedrine, Emde named it didesoxyephedrine. He considered II and III as possible structures for didesoxyephedrine and was able to reject III but not to prove II.
The introduction of desoxyephedrine as a drug some years ago has rekindled interest in I. The present paper is a report on attempts to throw some light on the reasons why Schmidt's and Emde's hydrogenation methods led to such divergent yields, and on the identity of didesoxyephedrine.
As a preliminary, it was ascertained that the same yield of 10% desoxyephedrine is obtained with I when hydrogenated with zinc-copper and hydrochloric acid as Schmidt obtained with the bromine compound. In subsequent experiments only I was used. The zinc-copper couple was then replaced by zinc alone in one experiment, by alkali-activated aluminum in another. Zinc afforded about the same yield as zinc-copper, while aluminum did not react.
On the other hand, calcium hydride, while without effect in itself, gave yields of desoxyephedrine comparable to those of Emde when used in conjunction with palladium, in acidic solution. Since a metal hydride in acidic solution is a source of molecular hydrogen3 [ H- + H3O+ = H2 + H2O ] the calcium hydride experiment amounts to a duplication of Emde's catalytic hydrogenation.
Table 1
Reduction of
1-Phenyl-1-Chloro-2-Methylaminopropane
| Reducing Agent | Yielda |
|
Desoxyephedrine |
Hydrocarbon |
|
| Zn + HCl | 12% | 77% |
| Zn-Cu + HCl | 10% | 74% |
| Zn-Pd + HCl | 34% | 42% |
| Zn-Cu-Pd + HCl | 26% | 46% |
| Al-Pd + HCl | 44% | 36% |
| H2-Pd + CH3COOHb | 80-90% | trace |
| CaH2-Pd + HCl | 86% | trace |
a Yields do not add up to 100% because some I
is inevitably hydrolyzed to a mixture of ephedrine
and pseudoephedrine.
b Yield reported by Emde1. For all other results
see experimental part of the present paper.
From these results it is plain that two different mechanisms must be at work, depending on whether hydrogen from zinc and acid or catalytically-excited molecular hydrogen is used as a hydrogenating agent. In the first case replacement of chlorine by hydrogen is a minor reaction, in the latter the principal one.
Next, the several metal-and-acid reductions were repeated in the presence of palladium catalyst in order to decide whether the faster rate of the reaction 2 H → H2 is responsible for the bad yields of the reaction R-Cl + 2 H → R-H + HCl. If so, then in the presence of the catalyst which reverses the reaction 2 H → H2, the same high yields should be obtained as with molecular hydrogen and palladium catalyst. Furthermore, the by-product hydrocarbon was isolated in every case and its quantity determined. The results of the above experiments are summarized in Table 1.
It is immediately apparent that the most important competitive reaction in the hydrogenation with metal and acid is not the conversion of atomic to molecular hydrogen but the conversion of I to a hydrocarbon, which cannot be suppressed even by increasing the concentration of atomic hydrogen by the catalyst. The identity of the hydrocarbon was not established at this point. As will be reported below, propenylbenzene was identified as the primary product of the reaction, in conformity with Emde's report; whether, and to what extent, it is further hydrogenated to propylbenzene as reported by Schmidt, is irrelevant to the reactions of I.
Scheme 1
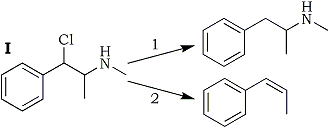
The results summarized in Table 1 may be expressed by Scheme 1; Path 1 being the main reaction with hydrogen and palladium as the hydrogenating agent, and Path 2 with metal and acid.
Obviously the presence of a metal has something to do with the predominance of Path 2. In order to obtain further information on its mode of action, I was reacted with zinc alone, without solvent and without acid. The reaction is almost instantaneous, strongly exothermic, and results in a good yield of propenylbenzene, without any identifiable side reactions.
This confirms the role of the metal, and we may interpret the findings reported above by the assumption that the zinc donates two electrons to the polar C-Cl and C-N bonds of I, allowing the ions Cl- and CH3NH- to break away, and leaving the biradical PhC·HC·HCH3 behind which is propenylbenzene when the orbitals of the two odd electrons overlap to form a π electron pair. Hydrogen, on the other hand, is not metallic enough to break the slightly polar C-N bond but it can break the C-Cl bond which in I - a substituted benzyl chloride - is very highly polar. Consequently a benzylic monoradical (IV) is formed, which quickly combines either with a hydrogen atom to form desoxyephedrine, or, to a lesser extent, with another radical IV to form II.
To test this hypothesis, I was reacted with copper, an element even less metallic than hydrogen. It might be reasonably expected to yield the monoradical IV which, in the absence of atomic hydrogen, can only dimerize. The expectation was confirmed and the product obtained showed the properties Emde reports for didesoxyephedrine. This may be considered as evidence for structure II for desoxyephedrine which Emde had suggested without being able to prove it.
Experimental
1-Phenyl-1-chloro-2-methylaminopropane hydrochloride (V) was prepared from ephedrine hydrochloride (Merck) and thionyl chloride, following the directions given by Emde1.
Hydrogenation with zinc and hydrochloric acid
V (22 g) was dissolved in 100 ml. of conc'd hydrochloric acid. To this solution 65 g. of zinc dust, moistened with water, was added. When the reaction subsided. it was kept going by adding more hydrochloric acid. When all the zinc was dissolved, the solution was distilled with steam as long as propenylbenzene came over, and was made alkaline and the steam distillation continued as long as the distillate was alkaline. The distillate was extracted with ether, the ether dried with sodium sulfate and saturated with HCl gas. The precipitated desoxyephedrine hydrochloride was washed with ether and dried; yield 2.3 g (12%), mp 171°C, [α]D25 +18° (c, 5, water). These data are in agreement with the physical constants reported by Emde4.
Hydrogenation with zinc in the presence of palladium and hydrochloric acid was performed in the same manner except that the zinc was previously immersed in a solution of 0.65 g of palladous chloride in 2 ml. of conc'd hydrochloric acid and 80 ml. of hot water, after a few minutes filtered. and washed with water.
The zinc-copper hydrogenation was carried out likewise but the zinc was first immersed in a solution of 50 g. of cupric sulfate in 1200 ml. of water until the blue color disappeared, filtered, and washed with water. For the reaction of zinc-copper in the presence of palladium, the copper-plated zinc was palladized as described above.
Hydrogenation with palladized aluminum and hydrochloric acid
Aluminum powder (Reynolds, 9 g) was washed successively with benzene, methanol, water, then immersed in 30 ml. of 0.1% NaOH solution. After two minutes. 100 ml. of water was added and the aluminum filtered and washed with water (activation by this method was chosen instead of the conventional amalgamation in order to avoid subsequent poisoning of the palladium). A solution of 0.2 g of palladous chloride in 200 ml. of hot water was then poured on the aluminum and left overnight. The palladized aluminum was filtered and washed with water and added to a solution of 22 g of V in a mixture of 200 ml. of conc'd hydrochloric acid and 200 ml. of water. The reaction was slow to start but became gradually quite vigorous and had to be moderated by outside cooling. When the reaction stopped, the unreacted aluminum was dissolved in conc'd hydrochloric acid, the mixture distilled with steam. and treated as before.
Hydrogenation with calcium hydride and hydrochloric acid in the presence of palladium
V (11 g.) was dissolved in 100 ml, of methanol. To this solution was added a solution of 0.25 g. of palladium chloride in 7.5 ml. of hot conc'd hydrochloric acid (Solution A). Calcium hydride (11 g) was covered with 100 ml. of methanol and solution A was added at such a rate that the temperature of the reaction mixture stayed at 35-40°C. with outside cooling if necessary. When the initially-vigorous hydrogen development subsided, enough conc'd hydrochloric acid was added to bring the pH to about 3 and the mixture was stirred during 1/2 hour. The clear solution was filtered from the palladium black and washed with 200 ml. of water. Then 250 ml. was distilled off to remove the methanol and whatever propenylbenzene might have been formed (no more than a trace was ever found), the residue made alkaline, and treated as in previous experiments.
The results obtained in the foregoing experiments are assembled in Table I.
Propenylbenzene from I and zinc
V (11 g) and 6 g of anhydrous sodium carbonate were suspended in 20 ml. of ether. 30 ml. of water was added, and agitated. The aqueous layer was washed with ether, the united ether solutions dried quickly with magnesium sulfate and filtered. The filtrate was kept under a vacuum until the ether had evaporated. Then 5 g. of zinc dust was added. The mixture heated up spontaneously to ebullition, and the reaction was moderated by cooling. The resulting liquid had a pure hydrocarbon smell and boiled at 173°C uncorr.; (bp of propenylbenzene 176°C, of propylbenzene 159.5°C). The unsaturation test was positive. N and Cl test negative; yield 4.8 g. (81.5%).
Didesoxyephedrine from I and copper
A preliminary experiment gave no results when the technique of the reaction with zinc was followed. Apparently the copper does not react vigorously enough to establish good contact with the liquid. Therefore the following method was used, 6 g. of anhydrous sodium acetate was dissolved in 80 ml. of water, and 14.4 g. of V was dissolved in the acetate solution. (These are the actual conditions of Emde's hydrogenation which yielded both desoxyephedrine and didesoxyephedrine.) Then 6 g. of copper powder was added and the mixture mechanically stirred during 12 hours. Excess alkali was added and the mixture distilled with steam to hydrolyze unreacted I, and to remove all possible volatile products (ephedrine, pseudoephedrine etc.). The residue was extracted with ether, the ether extract dried with magnesium sulfate. A yellow oil remained which crystallized slowly, yield 3.1 g (32%), mp 71°C (Emde reports 70°C). insoluble precipitate with mercuric chloride in HCl solution (Emde reports the same). The low yield was apparently due to incomplete reaction, since large amounts of copper also were found unreacted.
Summary
- In the reduction of 1-phenyl-1-chloro-2-methylaminopropane two competitive reactions are demonstrated, one being hydrogenation to desoxyephedrine, the other the formation of propenylbenzene by agents more metallic than hydrogen.
- The mechanisms of both reactions are discussed and interpreted by means of intermediate mono- and biradicals.
- The simultaneous formation of didesoxyephedrine (2,5-bis-methylamino-3,4-diphenylhexane) is interpreted as dimerization of the monoradical, and evidence for its structure is offered.