Introduction
Allylbenzene is a relevant precursor for the synthesis of amphetamines, just like safrole is one of the main precursors for the synthesis of MDMA. Aquiring Allylbenzene can be somewhat tricky, as it is a somewhat watched chemical because many chemical supply companies know what it can be used for. Unlike safrole, allylbenzene does not occur in large amounts in nature, but a chemical relative which does is cinnamaldehyde (3-phenyl-propenal).
The essential oil of cinnamon bark consists of about 90% cinnamaldehyde, and this can be isolated by vacuum distillation (bp 250°C at ordinary pressure). Do not use the oil of cinnamon leaves, because that consists chiefly of eugenol (70-90%). Cinnamaldehyde can also be had by the reduction of cinnamic acid or the oxidation of cinnamyl alcohol.
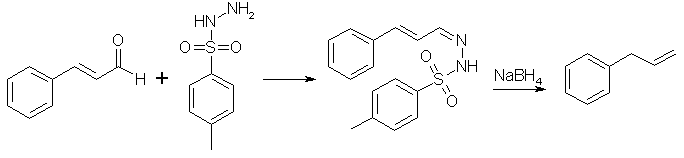
If we could somehow reduce the aldehyde group of cinnamaldehyde to a methyl group, propenylbenzene would be formed. This cannot be done directly though, but it is possible to condense cinnamaldehyde with para-toluenesulfonylhydrazide to form cinnamaldehyde tosylhydrazone, which in turn can be reduced by sodium borohydride or sodium cyanoborohydride to allylbenzene1. These procedure is described below.
A similar procedure, using bis(benzoyloxy)borane (made in situ from benzoic acid and BH3·THF) as the reducing agent2, boasts a 95% yield (85% isolated) of allylbenzene from cinnamaldehyde tosylhydrazone.
Experimental
Cinnamaldehyde tosylhydrazone
Mix 130ml of Cinnamaldehyde and 260ml of 95% ethanol in a 1L RB flask equipped with a reflux condenser. Then add 220g of p-toluenesulfonylhydrazide. Swirl the contents of the flask. Heat the flask at 100°C with magnetic stirring (or stirring of some kind). As the rxn progresses, the contents will become clear or at least translucent. Usually, the rxn takes 15 min from the time the temp of solution reaches 100°C until the end of rxn.
Now that the Cinnamaldehyde tosylhydrazone has been formed, let the solution begin to cool. As soon as the first crystals become apparent in the cooling solution, pour the mess into a beaker with a wide mouth. Once the solution has reached room temperature, the solution should be vacuum filtered to obtain the crystals. Once filtered place the crystals back in to a 600ml beaker and add 200-250ml of 95% ethanol. Bring to a slight reflux and then set aside to cool to room temperature. Do not place in a cooler environment to speed crystallization as this negates the recrystallization procedure.
Once cooled to room temperature, a mass of crystals should once again be apparent. Vacuum filter the crystals allowing air to pass through them until dry. Yield from pure aldehyde should approach 270g of the hydrazone. The yield from the oil will be proportionally lower based on the original aldehyde content. You can correct for the percentage of aldehyde in your oil to make the most of your p-toluenesulfonylhydrazide.
Allylbenzene (NaBH4 Method 1)
To a 5L RB flask, 2600ml of glacial acetic acid is added. The flask is then cooled to 0°C and 75g of sodium borohydride is slowly added with stirring. Attempt to keep the temperature in the range of 15-20°C. Then slowly add 226g of the cinnamaldehyde tosylhydrazone with stirring. For one hour after the completion of the addition, the stirring shall be continued at room temperature. Then the temperature will be raised to 70°C and the stirring continued for another 2.5 h. Nitrogen will be produced as the reduction of the hydrazone takes place.
Next pour the resulting solution onto 5 kg of ice. Allow the ice to melt and then add a 10% solution of NaOH with stirring until the solution is basic to red litmus paper. Extract the solution 4x200ml of a non-polar such as toluene or hexane. The solution is now dried with anhydrous CaCl2.
The dry solution is placed in an appropriate sized rb flask and fractionally distilled. If you did not dry the solution the toluene-water azeotrope will come over first. It will appear as a milky white solution. Then in the absence of water the toluene will distill over. Finally, regardless of procedure, the allylbenzene will come over at 156°C at ordinary pressure. Redistill the product for a final yield of approximately 40ml of allylbenzene.
Allylbenzene (NaBH4 Method 2)
In a 5L flask, place 2500ml of glacial acetic acid. Then add 225g of the cinnamaldehyde tosylhydrazone. Begin stirring at such a pace as to cause a slurry or emulsion. Then slowly add 280g of NaBH4 at such a rate as to prevent the froth formed from escaping the flask. Addition takes about an hour. Much more H2 gas is formed by this method and as such adequate ventilation must be provided to prevent accidents (explosion risk). After the addition of the sodium borohydride, the procedure continues as read above. Yield is approximately 60ml of allylbenzene (this is a 50% increase in yield).
Allylbenzene (NaBH3CN Method)
Into a container of around 10 L capacity put 2.5 L DMF and 2.5 L sulfolane. Mix them together, then add 300g of the tosylhydrazone derivative of cinnamaldehyde and 250g of sodium cyanoborohydride. Stir them into solution, then add a few tenths of a gram of the acid-base indicator bromocresol green. When the indicator dissolves, it will color the solution blue.
Next heat the solution to 105°C, then dropwise add concentrated hydrochloric acid until the pH drops below 3.8 as indicated by the color change of the solution from blue to tan. Then add 2 L of cyclohexane to the solution, and continue the heating with stirring for about an hour. I would think that toluene could be substituted for the cyclohexane. A little more bromocresol green is added during the heating period, and after one hour of heating, a little more hydrochloric acid is added dropwise to keep the pH below 3.8 as indicated by the tan color. Continue heating for an additional 1.5 hours, for a total of around 2.5 hours of heating.
After the solution has cooled, pour it into about 7 L of water, and shake the mixture around for a bit. Then separate off the organic layer floating on top of the water layer. Take the water layer, and extract it a couple of times with 200ml portions of cyclohexane or toluene. Add these extracts to the organic layer which had been separated off. Your product is in there, heavily diluted with solvent. Take the combined organic layer and solvent extracts, and wash this with about 1 L of water. Drain off the water layer, and repeat the water wash a couple more times. Distill off the solvent, then under a vacuum the allylbenzene to get about 100ml.