Summary
Since 1983 a large number of small-scale illicit laboratories producing morphine and heroin from commercially available, codeine-based pharmaceutical products have been encountered in New Zealand. The codeine demethylation procedure is based on the use of pyridine hydrochloride Very simple laboratory equipment and reagents are required and these can be utilised by people with little or no chemical background, following a recipe-like procedure. The process yields a characteristic product known as 'homebake'. This process is fully described.
Introduction
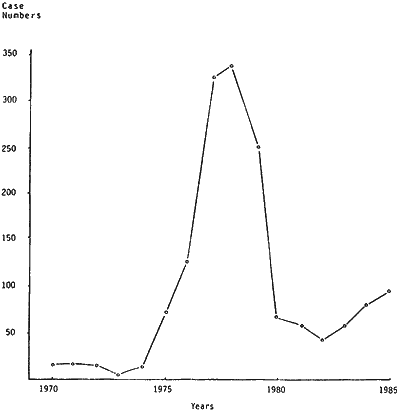
Fig. 1.
Illicit heroin cases submitted to Chemistry Division.
The patterns of drug abuse found in New Zealand (NZ) are influenced by the fact that it is a geographically isolated country with a small population (3.3 million). One recent feature has been the emergence of small-scale illicit laboratories producing morphine and heroin from codeine derived from commercially available codeine-based pharmaceutical products. This paper describes the background to this development, the methods used in such laboratories, and the approach taken by the forensic scientist in examining them.
In the late 1970s high quality South-East Asian heroin became widely available in NZ for the first time. Subsequent police operations in NZ, Australia and Britain led in 1980 to the collapse of the so-called 'Mr Asia' drug syndicate, responsible for much of this activity. Following this, and other successful NZ police and customs investigations, the amount of heroin available in NZ declined dramatically. This is illustrated in Fig. 1 which shows the number of illicit heroin cases submitted each year to the Chemistry Division of the NZ Department of Scientific and Industrial Research (DSIR).
Chemistry Division of the DSIR provides scientific support services to the police and customs in NZ. The resulting shortage, coupled with the established market for heroin, led to the development of a simple method for the production of morphine and heroin from codeine. Codeine, in certain compound products is not controlled under the NZ Misuse of Drugs Act (1975) and is available without prescription from retail pharmacies. The first laboratory using the process was seized in Auckland in January 1983. In the three years which followed to January 1986, Auckland police alone seized over 50 such laboratories, either fully functional or as disassembled 'kits', and over 90 were seized in NZ as a whole. Laboratories have been encountered in kitchens and bathrooms in most parts of the country. The home-made product of this simple recipe-like procedure has become known as 'homebake'. The equipment required for these laboratories is very simple and the product is normally made in small batches for the addict's own use, although there has been a trend towards the sale of samples of the end product.

Fig. 2.
The O-demethylation of codeine to yield morphine.
Sources of codeine
The codeine used in the homebake process may be derived from one of a number of possible sources. In a few cases codeine phosphate has been obtained from pharmacy burglaries. In most instances, however, it is derived from pharmaceutical products which may he purchased without a doctor's prescription. Those available in NZ containing the highest proportions of codeine are: 'Codral Forte' — 225 mg aspirin, 150 mg paracetamol, 30 mg codeine phosphate; 'Pirophen' — 325 mg aspirin, 325 mg paracetamol, 10 mg codeine phosphate. Awareness among pharmacists of the way in which these particular products are being misused has resulted in greater vigilance and has led to a wide variety of other preparations containing lesser amounts of codeine also being utilised.
The conversion of codeine to morphine

Fig. 3.
The relationship between heroin, morphine,
and the two monoacetylmorphines.
The O-demethylation of codeine to yield morphine (Fig. 2) has been reported using a variety of reagents including sodium propylmercaptide in dimethylformamide1 and boron tribromide in chloroform2. These methods require a considerable measure of chemical expertise and suitable laboratory equipment. The homebake laboratories have employed a very simple method based on the use of pyridine hydrochloride. This reagent was introduced to opiate chemistry by Rapoport and Bonner3 and applied to the conversion of codeine to morphine by Rapoport et al.4. Rapoport and Bonner carried out the reaction under nitrogen at a temperature of 220°C. Morphine is prone to decomposition or oxidation reactions at elevated temperatures and the nitrogen atmosphere prevents or reduces these. It also limits the access of moisture which reduces the yield.
The acetylation of
morphine to form heroin
In the acetylation of morphine to yield heroin (diacetylmorphine) the phenolic OH group on the 3-carbon is the first to react to give, as an intermediate, 3-O-monoacetylmorphine, which is further acetylated to heroin. Similarly, when heroin is deacetylated, either by hydrolysis or enzymatically, the more reactive 3-acetyl group tends to be removed first to yield 6-O-monoacetylmorphine as the predominant intermediate, although low levels of 3-O-monoacetylmorphine may also be formed under these conditions (Fig. 3)5.
Experimental Details
In this section the procedure followed in homebake laboratories is fully described. Additional comments on the method, based on studies carried out in this laboratory on the reactions involved, are included in the discussion section.
1. Extraction of codeine
Several packets of tablets, sufficient to yield about 2 g of codeine, are crushed and mixed with water. The mixture is filtered using a filter pump, Buchner funnel and side-arm flask, to remove tablet binding agents, diluents and other excipients. The aqueous filtrate is poured into a separating funnel and sodium hydroxide solution added to make the solution strongly alkaline. This is then extracted with chloroform (about 50 ml). The chloroform layer is drained off and evaporated to dryness using gentle heating (often on a domestic stove). The aqueous layer containing aspirin and paracetamol is discarded. The codeine base is recovered as a white crystalline solid for use in Step 3.
2. Preparation of pyridine hydrochloride
In a beaker pyridine (20 ml) and concentrated hydrochloric acid (25 ml) are strongly heated (to about 190°C) to drive off water. The product is cooled rapidly to form a waxy white solid which is stored in a sealed container in a freezer to minimise exposure to moisture and avoid decomposition.
3. Reaction of codeine and pyridine hydrochloride
The reaction is carried out using a boiling tube which is flame-dried before use. Pyridine hydrochloride (3.5 g) as prepared in Step 2 is then heated in the tube until it melts and any residual moisture is driven off. Any resulting condensation on the inside walls of the tube is wiped off. Codeine base (1.5 g) is added to the tube which is then stoppered with a rubber bung covered with filter paper and heated until the mixture starts to fume. Heating is continued until a reddish-orange colour develops in the reaction melt, which becomes noticeably more viscous (6-12 min). The contents of the boiling tube are then poured into a 500 ml separating funnel and the volume made up to 100 ml with water. Sodium hydroxide solution (10%) is added until the contents of the separating funnel are strongly basic. As the sodium hydroxide is added the contents turn milky-brown before becoming clear brown again. Chloroform (20 ml) is added. After extraction the greyish-brown chloroform layer is either discarded or put aside for later recovery of the codeine contained in it. The aqueous layer is poured into a 400 ml beaker and the pH is carefully adjusted to pH 9 using hydrochloric acid and narrow-range indicator paper. The solution is rapidly filtered under suction, using a Buchner funnel and 2 filter papers, to remove a fine, dark brown residue containing unwanted by-products. The filtered solution is then poured into a clean beaker and precipitation is induced by vigorously rubbing the side of the beaker with a 'seeding stick' as the pH is carefully lowered to 8.5 with additional hydrochloric acid. (In homebake laboratories a split wooden clothes peg is often used as the 'seeding stick'.) The product is allowed to settle for at least 5 min before being filtered off under vacuum. The morphine product is recovered as a powder, ranging in colour from beige to dark brown.
4. Conversion of morphine to heroin
Morphine powder as prepared in Step 3 is placed in a spoon. A small amount of acetic anhydride is added and the mixture ignited. Addition of acetic anhydride may be repeated. A brown or black tar-like residue remains.
Results and Discussion
1. Procedural details
The procedure outlined in the experimental section has been followed many times in this laboratory. The glassware and other equipment required for the reaction are remarkably simple and readily available from scientific supply companies. The main items include several beakers, a separating funnel, a filter pump and tap attachments, a Buchner flask and funnel, a boiling tube with a rubber bung or cork and an evaporating basin. The chemicals necessary include hydrochloric acid, pyridine, chloroform and sodium hydroxide. Acetic anhydride is also necessary if the final step to heroin is intended. Hydrochloric acid and sodium hydroxide are widely available. Chloroform, pyridine and acetic anhydride are available from chemical supply companies. Recent awareness among supply companies of the significance of a request for small amounts of these chemicals has led to stricter monitoring of orders. In a number of laboratories pyridine has been replaced by a crude mixture of picolines (methylpyridine isomers) and other substituted pyridines. In at least two cases the mixture also contained an intense purple dye indicating that it had been obtained from a chemical distributor where the mixture of pyridines and dye is added to ethanol in the course of preparing 'methylated spirits' (denatured alcohol).
The most common source of heat found in these laboratories has been small methylated spirits burners. These give a cooler flame than do laboratory bunsen burners and enable better control of the reaction between codeine and pyridine hydrochloride. In a few laboratories investigated, heating had been carried out using cooking oil on a domestic stove element. Under laboratory conditions the reaction has been carried out using a heated sand bath.
The use of a rubber hung in the boiling tube to produce a sealed reaction vessel is a simple solution to the problems of morphine oxidation and decomposition on heating and the need to minimise access of moisture to the reaction mixture.
2. Product yields
Homebake laboratory operators have claimed yields of morphine equivalent to 50% conversion from codeine but the reaction also forms a complex mixture of by-products, whose structures are now being studied by us. In our laboratory using these procedures nett yields have not exceeded 30%. Indeed, in the light of Rapoport and Bonner's work this appears to be the maximum that could be expected3. Morphine having a purity of 92% calculated as the anhydrous free base and determined by HPLC has been prepared, although purities in the 80% region are more typical. Negligible codeine is present with the morphine, indicating that the chloroform extraction step is efficient in removing this. This high purity, with little or no codeine contamination, is characteristic of 'homebake' morphine.
The crude method used to acetylate the resultant morphine can result in up to 60% conversion to heroin. The brown or black tar-like residue is used either by heating it strongly on a piece of aluminium foil and inhaling the fumes or by injection. If the injection route is used, water is added to the spoon, a small amount of acid may be added to assist dissolution, and the mixture warmed. The syringe is filled using a cigarette filter to remove insoluble by-products of the reaction. A distinctive feature of the product is that it contains unusually high levels of 3-O-monoacetylmorphine, clearly present because of incomplete acetylation of morphine (Fig. 3). ln complete contrast to the usual situation with illicit heroin, the 3-O-monoacetylmorphine level exceeds that of 6-O-monoacetylmorphine6. Recent studies have elucidated the requirements for the formation of 3-O-monoacetylmorphine and also its decomposition, since it is readily hydrolysed5,7-9. In homebake heroin the initial high 3-O-monoacetylmorphine level is rapidly reduced by this hydrolysis, particularly as the product is frequently dissolved in a small amount of water containing acid, in preparation for injection.
One completed synthesis, based on an initial quantity of approx. 2 g of codeine, yields an acetylated product which is dissolved in 4-5 ml of water. The concentration of heroin in this solution is typically between 10 and 40 mg/ml, depending on the skill of the operator. Obviously the solution also contains morphine and 3-O-monoacetylmorphine. This should provide enough for several 'fixes' or allow for a small surplus to be sold, depending on the level of addiction of the user.
Conclusions
Countering these homebake laboratories has proved to be a frustrating exercise for police and for forensic scientists called on to provide scientific support. The entire procedure from extraction of the codeine tablets through to the preparation of usable heroin solution can be completed by a practised operator in a few hours. The simplicity of the laboratory equipment allows easy portability. It has been known for operators to arrive at an address one afternoon and leave the next morning after having completed one or more syntheses. To support a charge of manufacturing morphine and/or heroin in court., the forensic scientist is asked by the prosecution to show that the required equipment and chemicals are present and, at least for key steps in the procedure, that they have been used. This frequently requires the determination of only trace amounts of products and by-products on the equipment seized. Laboratory operators have become aware of this and carefully clean and destroy vital evidence as they proceed. In some cases the equipment for the laboratory has been divided and kept in two places to prevent an operator being caught in possession of the full set.
On the other hand the problem is to some extent self-limiting. The laboratories are on a small scale, producing only enough product to satisfy the 'habit' of an individual addict and perhaps a few friends or clients. There are indications that the method does not lend itself to a large scale operation and the percentage yield drops significantly if attempts are made to increase the quantities involved. Although the original laboratory operators had chemical knowledge. subsequent operators have had to be taught the method. The percentage yield in the codeine to morphine conversion step is unpredictable and small variations in the experimental conditions at several crucial stages can make the difference between success. partial success and total failure. Experience is a big factor in judging the point of maximum yield in the reaction of codeine and pyridine hydrochloride and also in manipulation of the pH to obtain maximum recovery of the morphine product. Although heroin may be produced by this method more cheaply than it can be bought on the street in NZ. it is doubtful that the procedure would be persevered with if good quality imported heroin were readily available. A small number of similar laboratories have been encountered in Australia (Government Analytical Laboratories, pers. comm.) but because of the factors already discussed, it seems unlikely that the method described in this paper will become popular elsewhere.
In only one of the laboratories so far seized in Auckland has there been any indication of thinking beyond the pyridine hydrochloride based process. In that laboratory an unopened supply of boron tribromide was discovered. There via, no evidence of any attempt to use this reagent. Boron tribromide leads to a far more efficient conversion of codeine to morphine2, but it is felt that the expertise and laboratory equipment required are likely to continue to make it an unattractive alternative to the remarkably simple and readily available chemicals and equipment used in the homebake process.