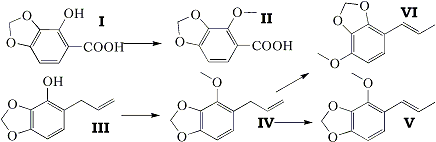
The naturally occurring substance croweacin has been proved to be 2-methoxy-3,4-methylenedioxyallylbenzene (IV). Ozonolysis of croweacin or oxidation of croweacin glycol with lead tetra-acetate gave 2-methoxy-3,4-methylenedioxyphenylacetaldehyde, and, in the former case, formaldehyde. The action of alcoholic potash on croweacin gave the propenyl isomer isocroweacin (V). The synthesis of croweacin was effected by the methylation of 2-hydroxy-3,4-methylenedioxyallylbenzene (III), an intermediate in the recently described synthesis of parsley apiole.
Croweacin, isolated from Eriostemon crowei (Crowei saligna) by Penfold and Morrison (J. Proc. Roy. Soc. N.S.W., 1922, 56, 227), has recently been shown by Penfold, Ramage, and Simonsen (J. Chem. Soc, 1938, 756) to be either 2-methoxy-3,4-methylenedioxyallylbenzene (IV) or the corresponding propenyl isomer (V), since croweacic acid obtained by oxidative degradation of the natural product with potassium permanganate was proved by synthesis to be 2-methoxy-3,4-methylenedioxybenzoic acid (II). The choice between the allyl (IV) and the propenyl isomer (V) was decided in favour of the latter, owing to the fact that a product, assumed to be the glycol, obtained from croweacin by oxidation with potassium permanganate under mild conditions was oxidised by lead tetra-acetate to 2-methoxy-3,4-methylenedioxybenzaidehyde.
During the course of entirely independent work on the synthesis of parsley apiole and derivatives (Baker and Savage, J., 1938, 1602) 2-hydroxy-3,4-methylenedioxybenzoic acid (I) and 2-hydroxy-3,4-methylenedioxyallylbenzene (III) had been prepared, and these substances were obviously extremely closely related to croweacic acid and croweacin. Accordingly 2-hydroxy-3,4-methylenedioxybenzoic acid (I) was methylated and yielded croweacic acid (II) identical with that prepared by the degradation of natural croweacin. Methylation of 2-hydroxy-3,4-methylenedioxyallylbenzene (III) yielded its methyl ether (IV), but the physical properties of this substance were sufficiently close to those recorded for croweacin itself to suggest that croweacin was actually this allyl derivative (IV) rather than the propenyl isomer (V). This view received support from a comparison of the refractive index of natural croweacin (nD20° 1.5329; Penfold and Morrison, loc. cit.) with the refractive indices of myristicin (nD20° 1.5403) and isomyristicin (nD45.5° 1.5655) and with those of other pairs of similar allyl and propenyl compounds (e.g., safrole, nD17.5° 1.5392, and isosafrole, nD18° 1.5786). Confirmation of the accuracy of this deduction was obtained by a comparison of dibromo-croweacin dibromide (Penfold, Ramage, and Simonsen) prepared from natural croweacin with the compound similarly prepared from the synthetic substance (IV), identity being established by a mixed melting-point determination.
Further proof that croweacin is correctly represented by the formula (IV) was obtained by ozonolysis of natural croweacin in methyl acetate solution. The products isolated were formaldehyde (as its 2,4-dinitrophenylhydrazone), 2-methoxy- 3,4-methylenedioxyphenyl- acetaldehyde (as its semicarbazone and 2,4-dinitro- phenylhydrazone) and 2-methoxy-3,4-methylenedioxyphenyl- acetic acid. Reinvestigation of the oxidation of natural croweacin with potassium permanganate showed that as well as the glycol (mp 91°C) and croweacic acid, croweacic aldehyde (mp 104°C) was also produced, and we are of the opinion that the previous production of croweacic aldehyde by oxidation of croweacin "glycol" (mp 97°C) with lead tetra-acetate (equivalent to 1/3 atom of oxygen) was due to the glycol consisting mainly of the aldehyde. This was confirmed by the failure of myristicin glycol to yield myristicic aldehyde under these conditions. careful purification of the genuine glycol has failed to raise the melting point above 91°C, and proof of its structure was afforded by oxidation to croweacic acid with excess of potassium permanganate at room temperature, and by oxidation with lead tetraacetate (1.1 mol) in acetic acid to 2-methoxy-3,4-methylenedioxyphenylacetaldehyde, isolated as its 2,4-dinitrophenylhydrazone. This derivative was identical with that prepared from the products of the ozonization of croweacin.
A difference in behaviour of synthetic and natural croweacin towards alcoholic potash established the fact that the former was not a homogeneous substance, and this may well account for the fact, previously mentioned, that their physical constants are not exactly the same. When natural croweacin was heated with alcoholic potash, it was converted into the liquid propenyl isomer isocroweacin (V), which was separated from a trace of sesquiterpene via the characteristic picrate, and which when oxidised with potassium permanganate yielded croweacic acid. When, however, the synthetic croweacin was submitted to the same treatment, it gave not only isocroweacin, but also a solid isomer, mp 64°C, in about 20% yield. This substance gave a dibromo-dibromide when treated with bromine in acetic acid, and when oxidised it gave, not croweacic acid, but 4-methoxy-2,3-methylenedioxybenzoic acid, thus proving it to be 4-methoxy-2,3-methylenedioxy- propenylbenzene (VI).
It was evident that in the preparation of (III) by rearrangement of the allyl ether of pyrogallol methylene ether, the usual o-migration of the allyl group had been accompanied by a p-migration, and hence the derived croweacin contained the position isomer corresponding to (VI). With the small amount of material at our disposal it has not been possible to prepare a homogeneous specimen of synthetic croweacin, as the accompanying isomer appears to possess a boiling point extremely close to that of croweacin itself. The non-homogeneity of (III) does not invalidate the synthesis of parsley apiole already recorded. The unusually large amount of p-migration of the allyl group which occurs in the molecular rearrangement of the allyl ether of pyrogallol methylene ether is noteworthy; it is probably due to the influence of the five-membered methylenedioxy ring. Isocroweacin behaves in an unexpected manner towards bromine in acetic acid at room temperature, with which it yields 1,2,3-tribromo-4-methoxy-5,6-methylenedioxybenzene with complete loss of the propenyl side chain.
Experimental
Oxidation of Natural Croweacin (IV)
(a) With ozone
A solution of the oil (3 mL) in methyl acetate (20 mL) at 0°C was ozonized until ozone was present in the issuing gases, which, during the oxidation, had been passed through water (A). The solvent was removed under diminished pressure and the residual gum was mixed with water (10 mL.) and heated on the water-bath for 1 hour and then on the sand-bath for 15 minutes under conditions permitting the escape of any readily volatile ketone (or aldehyde), the vapour being passed through a dilute acetic acid solution of p-nitrophenylhydrazine (B). The cooled reaction mixture, which contained a heavy brown oil, was extracted with ether, and the extract washed with aqueous sodium carbonate (C) and then with aqueous sodium hydroxide (2.5%) (D). The last solution was deep brown and there was obvious aerial oxidation. The ethereal extract, after washing with water, was dried; evaporation of the solvent left a mobile yellow oil (1.7 g). The oil was mixed with an excess of semicarbazide acetate; the semicarbazone, dec. 190-195°C, which formed very rapidly, was collected and washed with ether to remove a trace of resinous impurity.
2-Methoxy-3,4-methylenedioxyphenylacetaldehyde semicarbazone crystallised from alcohol in small rhombohedra, dec. 194-195°C, unaltered by further crystallisation. The 2,4-dinitrophenylhydrazone, prepared by digestion of the semicarbazone with an alcoholic solution of 2,4-dinitrophenylhydrazine sulfate, crystallised from alcohol-ethyl acetate in golden needles, mp 169-170°C. The aqueous solution (A) gave on addition of an aqueous solution of 2,4-dinitrophenylhydrazine sulfate a yellow precipitate of formaldehyde 2,4-dinitrophenylhydrazone, mp 165°C, both alone and in admixture. From (B), on dilution with water, a yellowish-brown solid separated ; this had mp 110- 120°C, but the quantity was too small for purification. Acidification of the sodium carbonate solution (C) precipitated an acid. This was shaken with ether, which left undissolved a considerable quantity of resin. Evaporation of the dried extract gave a brown, somewhat resinous oil (0.8 g), which slowly deposited needles. The crude acid was dissolved in aqueous sodium bicarbonate and filtered from resinous impurities (charcoal), the solution acidified, and the acid again taken up with ether, some resin remaining undissolved. The partly crystalline oil, which remained after removal of the ether, was digested with benzene, the filtered benzene extract evaporated, and the residue crystallised from cyclohexane and finally (twice) from hot water, 2-methoxy-3,4-methylenedioxyphenylacetic acid separating in long prismatic needles, mp 118-119°C. The sodium hydroxide solution (D) was immediately acidified and yielded a resinous phenol (0.15 g) which gave an intense brown ferric chloride reaction. It was not further examined.
(b) With potassium permanganate
Croweacin (10 mL) was oxidised with potassium permanganate under the conditions used by Penfold and Morrison (loc. cit., p. 230). The crude glycol (4.7 g) was crystallised (twice) from ligroin (bp 100-120°C) and finally from ether, from which it separated in nodules or rosettes of needles, mp 90-91°C after softening at 87°C. This mp was unchanged by sublimation in a low vacuum or by crystallisation from either cyclohexane or dilute methyl alcohol. The original mother-liquor was distilled in steam to remove the solvent; the residual oil (isolated by extraction with chloroform) gave a further quantity of the glycol on trituration with ether. The oil, which was soluble in ether, reacted with Brady's reagent to yield a 2,4-dinitrophenylhydrazone crystallising from ethyl acetate in red needles, mp 252-253°C, both alone and in admixture with 2-methoxy-3,4-methylenedioxybenzaldehyde 2,4-dinitrophenylhydrazone. The alkaline solution from which the glycol had been separated gave, on acidification, an acid (2.5 g), which crystallised almost completely and was identified as croweacic acid.
Oxidation of Croweacin Glycol
(a) With potassium permanganate
The finely powdered glycol (0.25 g, mp 90-91°C, prepared from natural croweacin) was suspended in water (25 mL.) containing some sodium hydroxide and shaken with powdered potassium permanganate at room temperature until a permanent pink colour was obtained. The filtered solution was concentrated and acidified; the acid which separated, mp 145-150°C, after crystallisation from hot water had mp 153°C, both alone and in admixture with croweacic acid.
(b) With lead tetra-acetate
The glycol (0.41 g) in acetic acid (20 mL, distilled over lead tetraacetate) was treated with lead tetra-acetate (0.9 g, 1.1 mol) with occasional shaking. After 24 hours, water (40 mL) and a solution of 2,4-dinitrophenylhydrazine (1 g) in dilute sulphuric acid were added; the orange precipitate (containing lead sulphate) was collected, washed, and extracted with boiling alcohol-ethyl acetate. The extracts yielded 2-methoxy-3,4-methylenedioxyphenylacetaldehyde 2,4-dinitrophenylhydrazone in bronze needles (0.2 g, mp 165-166°C), which, after recrystallisation from alcohol-ethyl acetate, formed fine, golden-yellow needles, mp 169-170°C, both alone and in admixture with the specimen previously described.
Isocroweacin (V)
Natural croweacin (4 g, bp 129-131°C/10 mmHg, was refluxed for 68 hours with potassium hydroxide (16 g) in alcohol (60 mL.), and the mixture diluted and submitted to steam-distillation. The colourless oil extracted from the distillate was twice distilled, head and tail fractions being neglected, and then had bp 145-147°C/12 mmHg. It was now treated with an equivalent of picric acid in hot alcoholic solution, and the picrate which separated was twice crystallised from alcohol; it formed garnet-red prismatic needles, mp 75-76°C. The isocroweacin regenerated from the picrate was unaltered in bp, but had nD20° 1.5675. It possesses a very weak odour of the croweacin type. It may be noted that croweacin yields no picrate.
1,2,3-Tribromo-4-methoxy-5,6-methylenedioxybenzene
Isocroweacin was treated with an excess of bromine in acetic acid solution (slight warming) and after 2 hours water was added, the solid was collected, washed with water and alkali, and crystallised twice from alcohol. The 1,2,3-tribromo-4-methoxy-5,6-methylenedioxybenzene formed rosettes of needles, mp 155-156°C both alone and m admixture with a specimen prepared by the bromination of 1-methoxy-2,3-methylenedioxybenzene (Baker, Montgomery, and Smith, J., 1932, 1282). In order to establish beyond all doubt that the propenyl side chain had been lost during the bromination process and not during the isomerisation, an examination was made of the physical properties of 1-methoxy-2,3-methylenedioxybenzene. It is a very easily crystallizable solid, mp 41°C, bp 112°C/17 mmHg.
2-Methoxy-3,4-methylenedioxybenzoic Acid (Croweacic Acid) (II)
2-Hydroxy-3,4-methylenedioxybenzoic acid (I) (Baker and Savage, loc. cit.), dissolved in a solution of potassium hydroxide (2 g) in water (5 mL) and acetone (5 mL), was warmed and shaken during the alternate addition of methyl sulphate (6 mL) and a solution of potassium hydroxide (2 g) in water (5 mL). After heating on the water-bath, the crude croweacic acid which was precipitated on the addition of hydrochloric acid was collected, washed, and crystallized from hot water (needles, mp 147-149°C, giving a very weak ferric chloride reaction). This acid was remethylated as before, and then separated from water in fine needles which gave no ferric chloride reaction, mp 152- 153°C, undepressed on admixture with a specimen of croweacic acid prepared from natural croweacin.
Synthetic Croweacin (IV), containing 4-Methoxy-2,3-methylenedioxyallylbenzene
2-Hydroxy-3,4-methylenedioxyallylbenzene (III) (Baker and Savage, loc. cit.) (2 g) (containing 4-hydroxy-2,3-methylenedioxyallylbenzene) in methyl alcohol (5 mL) was shaken during the alternate addition of a large excess of methyl sulphate and aqueous potassium hydroxide. The alkaline mixture was heated on the water-bath for 30 min and then distilled in steam. The colourless oil which passed over was extracted with ether, and the extract dried with sodium sulfate and distilled twice under diminished pressure. The product (1.7 g) had a constant bp 147-148°C/22 mmHg. It possessed a weak odour recalling that of safrole.
Dibromocroweacin Dibromide
The synthetic croweacin was brominated in acetic acid as described by Penfold, Ramage, and Simonsen. After precipitation with water the oily product was seeded with a trace of crystalline dibromocroweacin dibromide from natural croweacin, and after 12 hours the almost completely solid material was rubbed with a little light petroleum (bp 80-100°C), and crystallized from light petroleum (bp 40-60°C). It separated in prismatic needles, mp 102-104°C The mixed mp with a specimen of dibromocroweacin dibromide (mp 106-107°C) prepared from natural croweacin was 103-105°C, and the mixture completely solidified on cooling.
Action of Alcoholic Potash on Synthetic Croweacin: Preparation of isoCroweacin (V) and 4-Methoxy-2,3-methylenedioxypropenylbenzene (VI)
A mixture of synthetic croweacin (2 g), potassium. hydroxide (8 g), and alcohol (30 mL) was heated on the water-bath for 48 hours (the same result was achieved when heating was continued for a further 48 hours), diluted, and steam-distilled. A colourless oil and then a solid came over, and after several hours the solid was collected, thoroughly drained and dried. The filtrate (a) and solid (b) were separately examined.
The filtrate (a), which consisted of a milky suspension, yielded to ether isocroweacin as a colourless oil (1.2 g), which was twice distilled under diminished pressure; it had bp 150-151°C/15 mmHg., higher-boiling residue in the flask deposited on standing a trace of the solid isomer (b). The isocroweacin was identified by the characteristic picrate, mp 72-73°C, mixed mp with the picrate previously described, 73-75°C, and by conversion into 1,2,3-tribromo-4-methoxy-5,6- methylenedioxybenzene (see above), mp and mixed mp 155-156°C.
The solid (b) was crystallised twice from light petroleum (bp 40-60°C), in which it was easily soluble on warming. 4-Methoxy-2,3-methylenedioxypropenylbenzene (VI) (0.35 g) was obtained in long blunt-ended prisms, mp 84°C.
5,6,alpha,beta-Tetrabromo-4-methoxy-2,3-methylenedioxy-n-propylbenzene. Dibromo-dibromide of (VI)
Compound (VI) (0.1 g.) in acetic acid (2 ml) was treated with excess of bromine, and after 12 hours the crystalline tetrabromo-derivative (0.12 g) was collected, washed with acetic acid, and crystallised twice from alcohol, in which it was rather sparingly soluble. It separated in thin blunt-ended prisms, mp 115°C.
Oxidation of Synthetical isoCroweacin (V) and 4-Methoxy-2,3-methylenedioxypropenylbenzene (VI) to Croweacic Acid and 4-Methoxy-2,3-methylenedioxybenzoic Acid respectively.
Oxidation was carried out by shaking the propenyl compounds, dissolved in a little light petroleum, with excess of potassium permanganate at room temperature for 20 minutes. After passage of sulfur dioxide and removal of petroleum by boiling, the acids separated from the cooled solutions and were purified by crystallisation from water. The isocroweacin yielded croweacic acid (in the crude state accompanied by a little 4-methoxy-2,3-methylenedioxybenzoic acid), mp and mixed mp 153°C. The 4-methoxy-2,3-methylenedioxypropenylbenzene (VI) yielded 4-methoxy-2,3-methylenedioxybenzoic acid, mp 255-256°C (dec.) both alone and in admixture with the same acid prepared by Penfold, Ramage, and Simonsen.
Myristicin Glycol (2,3-Dihydroxy-3-methoxy-4,5-methylenedioxypropylbenzene)
To a mixture of myristicin (7.5 ml), water (500 ml), and crushed ice (500 g), were added potassium hydroxide (1.5 g) and then powdered potassium permanganate (11 g) in small portions with continual shaking. After completion of the reaction (10 minutes) the liquid was filtered, filtrate and residue extracted with chloroform, and the extracts dried and distilled, leaving an oil which solidified on standing (1.5 g). The glycol was crystallised twice from benzene and obtained in spherulitic bunches of fine needles, mp 90-91°C. The substance gave a large depression of the mp when mixed with croweacin glycol, mp 91°C.
Myristicinaldehyde 2,4-dinitrophenylhydrazone
Myristicinaldehyde was warmed with a dilute alcoholic solution of 2,4-dinitrophenylhydrazine sulphate. The bright red precipitate crystallised. from alcohol-ethyl acetate in dark brownish-red needles, mp 232°C after previous sintering.