Summary:
Various N-benzyl derivatives of amino acids and amines were deprotected to the corresponding free amino acids and amines using ammonium formate as the hydrogen source.
Catalytic transfer hydrogenation has been successfully applied for removal of a benzyl group from protected benzyloxycarbonyl, benzylester and benzylester derivatives of peptides and amino acids using cyclohexene3,4, 1,4-cyclohexadiene5, hydrazine-hydrate6 and ammonium formate7,8 as the hydrogen donor. Deprotection of the N-benzyl group, however, is still most often carried out by traditional high pressure catalytic hydrogenation9,10. Recently, B. El Amin. et al.11 reported that removal of a benzyl group from Z-amino acids using formic acid as the hydrogen donor provides formate salts of amino acids as end products instead of free amino acids.
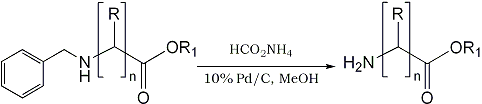
In our on-going program to develop rapid synthesis of radio-labeled tracer molecules for Positron Emission Tomography (PET), we are interested in the radioisotopic synthesis of 11C- amino acids (11C-half life=20.4 min) such as [11C-carboxyl)-γ- aminobutyric acid, [11C-carboxyl]-β-alanine, etc. via N-benzyl derivatives of bromoalkanes. In this paper we wish to report a rapid deprotection of the N-benzyl group to the corresponding free amino derivatives using ammonium formate as shown in Scheme I (R = H/Alkyl; R1 = H/C2H5; n = 1-3).
Experimental:
A typical procedure for debenzylation is as follows. To a stirred suspension of an appropriate N-benzyl compound (3 mmol) and an equal weight of 10% Pd-C in dry methanol (20 ml). anhydrous ammonium formate (15 mmol) was added in a single portion under nitrogen. The resulting reaction mixture was stirred at reflux temperature and the reaction was monitored by TLC. After completion of reaction, the catalyst was removed by filtration through a celite pad, which was then washed with 20 ml of chloroform. The combined organic filtrate, on evaporation under reduced pressure, afforded the desired amino derivative. In the case of free amino acids. the reaction mixture was filtered while hot and the celite pad was washed with boiling water (20 ml). Characterization of this new procedure is shown in Table 1.
| N-Benzyl Compounds (Bz=CH2C6H5) |
Productsb | Reaction Time (min) |
Yield a (in %) |
Rel. Rf Valuesc |
| (CH3)2CHCH2CH(CO2H)NHBz | (CH3)2CHCH2CH(CO2)NH3 | 6 | 96 | 0,47f |
| CH3CH2CH(CH3)CH(CO2H)NHBz | CH3CH2CH(CH3)CH(CO2)NH3 | 8 | 95 | 0,49f |
| BzN(CH2CO2H)2 | NH(CH2CO2H)2 | 10 | 64 | 0,24f |
| BzNHCH2CO2C2H5 | NH2CH2CO2C2H5 | <10 | 97 | 0,50e |
| BzNH(CH2)3CO2C2H5 | NH2(CH2)3CO2C2H5 | 6 | 95 | 0,39e |
| Ethyl N-benzylnipecoate | Ethyl nipecoate | 10 | 91 | 0,31e |
| N-Benzyl-2-methylimidazole | 2-Methylimidazole | 60 | 97 | 0,18d |
- Unoptimized. Isolated yields are based on a single experiment.
- characterized via comparison with authentic samples (IR, 1H-NMR, TLC and mp).
- relative Rf value = Product TLC Rf/starting material TLC Rf,
Merck silica gel plates, mobile phase: CHCl3:MeOH:58% NH4OH. - 9:1:3 drops.
- CHCl3:MeOH (96:4).
- BuOH:AcOH:H2O (4:1:1).
In most cases, the reaction is over within 6-10 min: however, for N-benzyl-2-methylimidazole. the reaction requires 60 min for completion. These results demonstrate a rapid and versatile system for removal of an N-benzyl group from a wide variety of compounds including protected amino acids under moderate reaction conditions.