Abstract
The synthesis of the deuterium labeled, endogenously occurring, indolealkylamine hallucinogens N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine via reduction of amide intermediates with lithium aluminum deuteride is described. The compounds were characterized with 1H-, 2H- and 13C-NMR. These compounds were synthesized for use as probes for investigating the metabolism of these compounds by MAO via the in vivo kinetic isotope effect.
Introduction
The indolealkylamine hallucinogens N,N-dimethyltryptamine (DMT, 4) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) have been identified as normal constituents of human blood1-6, urine7-10, cerebrospinal fluid11,12 (CSF) as well as in a variety of plant species. DMT has also been identified as a putative neurotransmitter or neuromodulatory substance in rat brain13. The indole-N-methyltransferase enzymes capable of synthesizing DMT and 5-MeO-DMT from tryptamine derived from L-tryptophan and S-adenosyl-methionine have been described and characterized in human lung, brain, blood and CSF and in various mammalian species14.
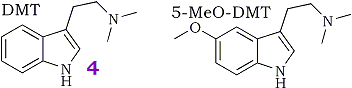
Numerous groups have attempted to relate mental disorders such as schizophrenia to high brain concentrations of these compounds resulting from perhaps a metabolic error, but a clear relationship between the two has not yet been delineated. However, the in vivo production of these interesting compounds strongly suggests that they serve some physiological role which is not yet understood.
For a number of years our group has been interested in studying the pharmacological properties of DMT and related indolealkylamines, in particular the in vivo metabolism. Indole-3-acetic acid (IAA) has been identified as the major in vivo metabolite of DMT. IAA is formed presumably via rapid oxidative deamination by monoamine oxidase (MAO) and is then excreted in urine15. These studies are in good agreement with more recent studies which have shown that DMT is essentially cleared from whole rat brain in ca. 30 min and that DMT is undetectable in the liver and blood plasma after 30 min16. The dominance of the deamination pathway makes it difficult to study the minor metabolites. Traditionally this difficulty has been surmounted by pre-treating animals with a MAO inhibitor such as pargyline. Inhibition of the major catabolic route leads to "shunting" to the minor metabolic routes, facilitating the study of the minor metabolites. Unfortunately pre-treatment of animals with pargyline can give experimental results which are difficult to interpret since pargyline also inhibits the N-oxidation and demethylation of DMT by 90%17. This observation suggests that the reported potentiation of the behavior-disrupting effects and the reported tissue levels of DMT measured in animals pre-treated with pargyline may not have been solely due to MAO inhibition.
Mechanistically, the deamination step presumably involves abstraction of an α-proton in the rate determining step of the reaction followed by deamination. This assumption led our group to speculate that substitution of the α and β protons of the ethylamine side-chain with deuteriums would produce the same effect via an in vivo kinetic isotope effect as pre-treatment with an appropriate MAO inhibitor (3). Thus a 2H-labeled compound would be an attractive alternative to pre-treatment with a MAO inhibitor for metabolic studies.
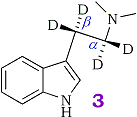
The results of the metabolism study using α,α,β,β-tetradeutero-N,N-dimethyltryptamine ([2H]4DMT, 3) have been published18. The labeled compound was metabolized at a significantly slower rate than proteo-DMT (4) and has indeed proved useful as a metabolic probe for studying the minor metabolic products. Although we concluded that the α-deuteriums were responsible for the observed isotope effect it is impossible to measure the contribution of the α-deuteriums alone, since the ethylamine side-chain was fully deuterated. Although the β-deuteriums are not involved mechanistically, it has been demonstrated in a similar system that the β-deuteriums of aromatic ethylamines change the rate of deamination and produce a small rate enhancement. We are now interested in demonstrating unambiguously that the α-position is responsible for the observed effect and is the only position involved in the deamination step. For this reason, our present study requires compounds labeled only in the α-position.
In this paper the synthesis and spectral properties of dimethyltryptamine (7) and α,α-[2H]2-5-methoxy-N,N-dimethyltryptamine (10) and a convenient synthesis of [2H]4DMT (3) is presented. Complete 1H-, 2H- and 13C-NMR assignments for [2H]4-5-MeO-DMT (11, isotopic purity of 97.5%) are also described19.
Results and discussion
Benington and Morin previously synthesized 3 in four steps from indole for use as an internal standard, however, its synthesis was not reported20 In their synthesis indole was acylated with oxalyl chloride to give the 3-substituted indole which was immediately reacted with etherial dimethylamine to give the keto-amide (2). Reduction of the keto-amide with lithium aluminum deuteride (LAD, LiAlD4) gave (3). Our group now utilizes commercially available indole-3-glyoxylic acid which affords the compound of interest in three steps. In a one-pot reaction the acid is converted to the acid chloride 1 with thionyl chloride which is not isolated but is immediately converted to the same keto-amide 2 by saturating the solution with dimethylamine gas. In our hands, the preparation of 1 from the acid required low temperature and low concentration in order to prevent highly colored by-products. Attempts to isolate the acid chloride for analysis failed. [...]
Reduction of the keto-amide 2 with LiAlD4 gave 3 which was readily purified by sublimation under diminished pressure. For spectral comparisons, we also reduced a small amount of 2 with LiAlH4 to give proteo-DMT (4). [...] Reaction of indole-3-acetic acid with thionyl chloride gave the acid chloride 5 which was reacted with dimethylamine to give the amide 6. Reduction with LiAlD4 afforded 7 which was purified by sublimation. [...] Using the same procedure, 5-methoxyindole-3-acetic acid was converted to the acid chloride 8 which was then converted to the amide 9. Usual reduction and purification afforded 10. [...]
Experimental
General Methods
All reagents and solvents were of the highest available purity and were used without further purification. Lithium aluminum deuteride (98%) was obtained from Aldrich Chem. Co. Thin layer chromatography (TLC) analyses were performed on Kieselgel aluminum backed silica gel 60 F254 plates (0.2 mm) obtained from E. Merck and were visualized using an ultraviolet light (254 nm) or I2.
2-(3-Indolyl)-glyoxal Chloride (1)
A solution of indole-3-glyoxylic acid (1.0 g, 5.3 mmol) in ether (100 mL) was stirred and cooled in a dry-ice bath for 15 min. SOCl2 (2.0 mL, 17 mmol) was then gradually added to the solution. TLC analysis (MeOH/PhMe, 10:90) of the mixture showed complete disappearance of indole-3-glyoxylic acid after 1 h and formation of a higher running compound with Rf 0.39. The product was diluted with a large amount of dry ether and used directly without purification.
2-(3-Indolyl)-N,N-dimethylglyoxalamide (2)
Dimethylamine gas was passed through the etherial solution of 1 for 3-5 min and the reaction mixture was stirred continuously for an additional 20-30 min. Excess solvent was removed to give 2 (0.89 g, 4.1 mmol) as a solid in 77% yield based on indole-3-glyoxylic acid, mp 184-185°C (lit.19 184-185°C).
α,α,β,β-[2H]4-Dimethyltryptamine (3)
To a stirred suspension of LiAlD4 (0.1 g, 2.4 mmol, 98%) in dry ether (8 mL) was gradually added the amide 2 (0.1 g, 0.46 mmol) in CH2Cl2 (5 mL). The mixture was refluxed for 3-4 h in an oil bath, cooled in an ice bath, and treated with several drops of water to decompose excess LiAlD4 reagent. The reaction was vacuum filtered to remove any remaining solids, dried (MgSO4), and solvents removed. The yield was 67% (0.06 g, 0.31 mmol), mp 47-49°C. Isotopic purity 94%.
N,N-dimethyltryptamine (4)
LAH reduction of 2 gave 4 in 76% yield (0.066 g, 0.35 mmol), mp 44-46°C (lit.19 45-46°C).
2-(3-Indolyl)-acetyl chloride (5)
Indole-3-acetic acid (2.0 g, 11.4 mmol) was converted to the acid chloride (ether, 200 mL; SOCl2, 2.0mL, 17 mmol) using the procedure described for the synthesis of 1.
2-(3-Indolyl)-N,N-dimethylacetamide (6)
Acid chloride 5 was converted to the amide 6 using dimethylamine as described for the synthesis of 2. Sublimation under diminished pressure gave 1.6 g (7.9 mmol, 69%) based on indole-3-acetic acid, mp 117-119°C.
α,α-[2H]2-N,N-Dimethyltryptamine (7)
LiAlD4 (0.1 g, 2.4 mmol, 98%) was suspended in dry ether (8 mL). The mixture was stirred and the amide 6 (0.25 g, 1.24 mmol) in CH2Cl2 (100 mL) was added over 5 min. The reaction was refluxed for 2-3 h in an oil bath at which time TLC analysis (MeOH) indicated disappearance of 6 and formation of a new spot at the origin. Workup as described for the synthesis of 3 and sublimation gave 0.16 g (0.84 mmol) of 7 in 68% yield, mp 44-46°C (lit.19 mp 44-46°C). Isotopic purity 97%.
2-(5-Methoxy-3-indolyl)-acetyl Chloride (8)
5-Methoxyindole-3-acetic acid (0.5 g, 2.44 mmol) was converted to the acid chloride (CH2Cl2, 100 mL; SOCl2, 1.0 ml, 8.5 mmol) using the procedure described for the synthesis of 1.
2-(5-Methoxy-3-indolyl)-N,N-dimethylacetamide (9)
The acid chloride 8 was diluted with CH2Cl2 and immediately treated with dimethylamine gas. The excess solvent was removed and the crude product sublimed under diminished pressure. The yield was 74% (0.42 g, 1.8 mmol) based on 5-methoxyindole-3-acetic acid, mp 78-80°C.
α,α-[2H]2-5-methoxy-N,N-dimethyltryptamine (10)
A suspension of LiAlD4 (0.1 g, 2.4 mmol, 98%) in dry ether (8 mL) was stirred and the amide 9 (0.25 g, 1.08 mmol) in CH2Cl2 (10 mL) gradually added. The mixture was refluxed for 2-3 h in an oil bath and then cooled to room temperature. Usual workup and purification gave 10 in 68% yield (0.16 g, 0.73 mmol) based on the amide 9, mp 49-51°C. Isotopic purity 99.7%.