Notes on the use of 1-dimethylamino-2-nitroethylene (DMANE)by RhodiumIf you direct your attention to J. Org. Chem. 42, 1784 (1977), you will discover the reagent 1-dimethylamino-2-nitroethylene (DMANE), which is easily prepared from the inexpensive reagents DMF, dimethylsulfate, nitromethane and sodium ethoxide. The reagent is capable of directly adding the 2-nitrovinyl (-CH=CH2-NO2) side chain to the 3-position of indole, as well as a variety of substituted benzenes. Do you have some 4-acetoxyindole lying around, but don't want to go through the hassle of using oxalyl chloride when making a 4-hydroxy tryptamine out of it? Then turn to DMANE, which is dissolved in TFAA, and the 4-acetoxyindole dumped right in. Ten minutes later you return, remove the solvent and let your product 3-(2-Nitrovinyl)-4-acetoxyindole crystallize in almost 80% yield, ready to become reduced to 4-hydroxytryptamine. We can also imagine that you are sitting with some 2,5-dimethoxyphenyl propyl sulfide, and want to turn it into a juicy phenethylamine. You can either subject it to a vilsmeyer formylation, followed by a Knoevenagel condensation with nitromethane to give you the nitrostyrene, or - you can simply get it in one step with this reagent. If you turn down this reagent with the excuse that you don't want any low potency phenethylamines, I can tell you about the homolog you can use to create phenylnitropropenes just as easy - 1-Dimethylamino-2-nitropropene. Below, I have included a few examples of the reagent, applied to various indole substrates, and even further down, you'll find preparation details of both the above-mentioned nitro reagents, as well as how to prepare a DMF/Me2SO4 complex needed for doing it. There is one downside to the procedure, and that is that the preferred solvent for the reaction seems to be trifluoroacetic acid. The authors of the above article mentions some italians who were using the reagent in ethanolic HCl (but with relatively low yields), so TFAA is at least not absolutely needed. 3-(2-Nitrovinyl)indoleTo a stirred solution of 1-dimethylamino-2-nitroethylene in trifluoroacetic acid (15 mL) at ice-bath temperature was added 3.51 g (30 mmol) of indole and the mixture was stirred in a nitrogen atmosphere for 10 min. During this time the color of the solution changed from light yellow to dark. The mixture was then allowed to warm up to room temperature and was poured into ice water (300 mL), from which a yellow semisolid precipitated. The aqueous solution was extracted with ethyl acetate (350 mL) and then twice with the same solvent (100 mL). The combined organic phases were washed with saturated NaHCO3 solution (150 mL) and with saturated NaCl solution (100 mL) and dried (Na2SO4). Removal of solvent in vacuo afforded 5.40 g (96%) of a yellow solid. Recrystallization from hot methanol gave yellow prisms: mp 172°C (lit 171°C). Ref: JOC 42, 1784 (1977) 3-(2-Nitrovinyl)-5-methoxyindoleA 15-mL flask equipped with reflux condenser was charged with 580 mg (5 mmol) of 1-dimethylamino-2-nitroethene and 3 mL of trifluoroacetic acid. To this stirred solution was added 735 mg (5 mmol) of 5-methoxyindole. The resulting suspension was heated to 30-45°C for 10 min and the solution was then allowed to cool. The dark slurry was poured into ice water. Extraction with ethyl acetate was followed by washing of the organic layer with saturated NaHCO3 and saturated NaCl solution. After drying (Na2SO4), the solvent was evaporated, yielding 1.1 g of dark green crystals. Purification by column chromatography (100 g, silica gel, CH2Cl2) yielded 60 mg (8%) of unreacted 5-methoxyindole and 697 mg (64%) of 3-(2-nitrovinyl)-5-methoxyindole. Recrystallization from acetone/hexane gave yellow needles: mp 162-165°C (lit 157-158°C). Ref: JOC 42, 1784 (1977) 3-(2-Nitrovinyl)-4-acetoxyindole
To a solution of 175 mg (1.0 mmole) of 4-acetoxyindole in 1 ml of trifluoroacetic acid was added 116 mg (1 mmole) of 1-dimethylamino-2-nitroethylene. The reaction mixture was heated at 55°C under a nitrogen atmosphere for 10 minutes during which time the light yellow solution became dark red. The reaction was cooled to room temperature and the solvent distilled under reduced pressure. The resulting red oil was dissolved in 2 ml of chloroform and the solvent removed in vacuo. This was repeated with 3 ml of dioxane. Addition of 1 ml of ethyl acetate and scratching induced crystallization of the residue. Hexane (0.25 ml) was added and the yellow needles collected by filtration and dried in vacuo to give 191 mg of 3-(2-Nitrovinyl)-4-acetoxy-indole (78%), mp 200-203°C (dec). Ref: J. Heterocyclic. Chem. 18, 175 (1981) 3-(2-Nitrovinyl)-4-acetoxyindole (335 mg, 1.36 mmoles) was dissolved in 4 ml of tetrahydrofuran and added to a stirred suspension of 300 mg (7.89 mmoles) of lithium aluminum hydride in 5 ml of tetrahydrofuran. After the addition the reaction was refluxed for 20 minutes, cooled to room temperature, and water added dropwise until gas evolution ceased. The mixture was filtered (Celite, dry nitrogen atmosphere) and the filtrate concentrated under reduced pressure. The resulting clear syrup (slight blue tinge) was dissolved in a mixture of 5 ml of pyridine and 3 ml of acetic anhydride. After standing overnight at room temperature the solvents were distilled under reduced pressure and the residue purified by preparative thin-layer chromatography in 5% methanol in chloroform. The eluted (ethyl acetate) product was crystallized from ethyl acetate/hexane to give 94 mg (26%), mp 150°C. Ref: J. Heterocyclic. Chem. 18, 175 (1981) N,N-dimethylformamide and dimethylsulfate complex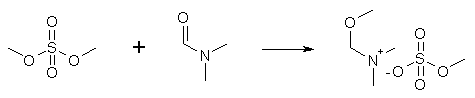
The reaction between N,N-dimethylformamide and an equimolar amount of dimethylsulfate (which takes two days at room temp, and two hours at 60-80°C, with no solvent added) furnishes the O-methyl complex of the amide. The formation of the complex is revealed through a change in viscosity and refraction index. Both DMF and Me2SO4 are soluble in ether, benzene and ethyl acetate, but their complex isn't. Upon attempted distillation of the complex, the components are re-formed. Ref: Angew. Chem. 73, 493 (1961) 1-Dimethylamino-2-nitroethylene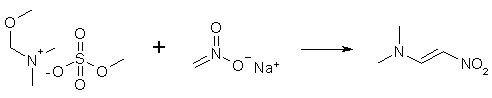
To a solution of 2.3 grams of sodium metal in 100 ml of absolute ethanol, 20g of DMF/Me2SO4 complex and 6.1 grams of nitromethane is added. The mixture is heated to a boil for 1-2 minutes, cooled to room temperature, and the solvent was then evaporated in vacuum. The mixture is then extracted with methylene chloride, the solvent is again evaporated in vacuum, and the residue is washed with cold isopropanol, and then recrystallized from the same solvent. The yield is 60% of melting point 104°C. Ref: Ber 98, 3847 (1965) 1-Dimethylamino-2-nitropropeneA solution of 10 grams of sodium metal in 500ml of absolute ethanol is cooled to 10°C and 90 grams of DMF/Me2SO4 complex and 47.5 grams of nitroethane is added. The solution is shaken with cooling for five minutes, and the solution takes on a orange-red color. The solvent is removed at 30-35°C in a rotavap, and the solid yellow residue is extracted with methylene chloride, and the solvent is again removed under vacuum. The orange-red oily residue is cooled in the fridge, whereupon it crystallizes. The crystals are washed with a small amount of cold ether, and are recrystallized from ethanol to give yellow crystals with a melting point of 78°C in a yield of 73% of theory. Ref: Ber 107, 1499 (1974) Addendum by Cesium: 1-dimethylamino-2-nitroethylene CA SEARCH 1982-7/2000 100:22856 The chemistry of indoles. XIX. Synthetic study directed toward cyclopiazonic acid. Chem. Pharm. Bull., 31(6), 2153-6 (English) 1983. 97:216537 Psilocine analogs. III. Synthesis of 5-methoxy- and 5-hydroxy-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indoles. J. Heterocycl. Chem., 19(4), 845-8 (English) 1982. 96:104201 Synthesis of 1H-[1,2]diazepino[4,5-b]indole derivatives. J. Heterocycl. Chem., 18(5), 889-92 (English) 1981. 120:77615 2-Substituted 5-methoxy-N-acyltryptamines: synthesis, binding affinity for the melatonin receptor, and evaluation of the biological activity. J. Med. Chem., 36(25), 4069-74 (English) 1993. 121:9157 Tryptamines for treatment of circadian rhythm disorders. EP 93-830269 24 Jun 1993 Unfortunatelly, all with trifluoroacetic acid as a solvent :-( |