Although the constitution of ephedrine (III) is no longer in doubt, interest in this base has been renewed by the pharmacological work of K. K. Chen.1 Since it seemed not improbable that other bases analogously constituted might possess more desirable or at least interesting physiological properties,2 the authors entered this field with a view to the improvement of known methods of synthesis or to the discovery of new ones.
- C6H5C=(NCH3)COCH3
- C6H5COC=(NCH3)CH3
- C6H5CH(OH)CH(NCH3)CH3
It was observed that a solution of 1-phenyl-1,2-propanedione [C6H5COCOCH3],3 in petroleum ether reacts exothermally with dried gaseous methylamine to form a colorless crystalline compound with the elimination of water. This substance is unstable and gradually decomposes to an indefinite mixture. Treatment with acids regenerates the two components and titration shows that approximately one molecule of amine is combined with one of the diketone. Aside from purely speculative formulas for this compound only two (I and II) need be mentioned, and of these the former may at once be disposed of on purely theoretical grounds.
The considerable affinity demand of the phenyl group4 leaves the α-carbon relatively saturated so that any negative ions (CN-, HSO3- etc.) have a tendency to attach themselves to the β-carbon in preference. Furthermore the effect of the carbonyl groups is reciprocal (the ketone group of ethyl pyruvate is more reactive than those of acetone, ethyl acetoacetate, or ethyl levulinate),5,6 that is, each enhances the additive capacity of the other. Finally, methylbenzyl ketone reacts readily with bisulfites, whereas ethylphenyl ketone does not, and the former is more reactive than acetone,6 so that the phenyl group enhances the additive capacity of the β-carbon, an effect in accord with the Flürscheim's theory of alternate affinity demand.4
Sufficient experimental proof of the correctness of Formula (II) is found in the fact that catalytic reduction leads to ephedrine (III), that is, the carbonyl group is reduced to a secondary alcohol and the imino double bond (-C=N-) is reduced at the same time.
It is interesting to note that only a very small proportion of pseudo-ephedrine accompanies the main product, dl-ephedrine. The method of Eberhardt7 yields a considerable amount of the pseudo-form and that of Späth and Göhring8 yielded the pseudo-form almost exclusively. To offer an explanation for this observation the authors assume that there is a repulsion between the N- and the ketonic O-atoms, their mean positions in space being on opposite sides of the carbon chain, so that the reduced product also tends to have the HO- and CH3NH-groups distant from each other, a postulate already advanced on other grounds for ephedrine. Conversely, it has been proposed that these groups are spatially adjacent in pseudo-ephedrine.9
Experimental
Preparation of dl-Ephedrine
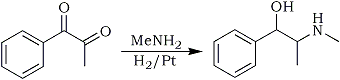
A mixture of 50 mL of absolute ethyl alcohol, 7.4 g of methylphenyl diketone (0.05 mole) and an alcoholic solution of methylamine containing 1.6g (0.05 mole) was reduced catalytically with hydrogen in the presence of 0.1 g of platinum oxide10. In some experiments there was a long induction period and then the yield was low. This behavior could be obviated by reducing the catalyst first and then adding the reactants. When reduction no longer proceeded, the catalyst was removed by filtration and about half the alcohol removed under reduced pressure. By this means any excess of methylamine was removed. The solution was made just acid with alcoholic hydrogen chloride and evaporated to dryness. The solid hydrochloride was washed with cold acetone and dried. A small amount of pseudoephedrine hydrochloride could be extracted from this by means of hot chloroform and by working up a number of extractions sufficient was obtained for definite identification (mp 164°C; free base, mp 118°C)8.
The dl-ephedrine hydrochloride was purified by recrystallizing once from alcohol-acetone and melted at 189°C8. The free base was recrystallized from chloroform-petroleum ether and melted at 75°C. The yield was 2.5-4.0 g.
Summary
- A new reaction has been discovered whereby it is possible to synthesize the alkaloid ephedrine.
- This reaction consists in taking advantage of the differential additive capacity of the two ketonic groups in alkylaryl o-diketones.
- 1-Phenyl-1,2-propanedione condenses with one molecule of methylamine and the condensation product on reduction yields dl-ephedrine.
- Several homologs of ephedrine have been synthesized by this method and their description will form the subject of an early communication.