In a usual laboratory day, the greatest share of working time is devoted to sample isolation and purification. Usually, chromatographic separation plays a central role in this effort. We outline here a procedure for column chromatography that is both efficient (mixtures showing ΔRf = 0.05 by TLC are routinely separated) and easily scaled up.
There are two central concerns in column chromatography: packing of the absorptive bed and sample application. This procedure, a modification of the short-column technique,2 effectively addresses both of these concerns. The steps to follow in packing a column are detailed in Figure 1. Note that the silica gel bed is first allowed to settle by gravity flow and then further compacted by application of air pressure.3,4 This assures a dense, evenly packed bed. Then, rather than application of the mixture to be chromatographed in liquid form, it is first evaporated onto coarse silica gel.5 This assures even application of the sample on to the top of the column and avoids concerns about mixtures that are not soluble in the (usually nonpolar) column solvent.
We find it convenient in running such columns to adjust the air pressure so as to collect about one fraction per minute. Fractions are monitored by TLC. For routine separations, the polarity of the eluant is adjusted so that the first component of the mixture appears in about fraction 10. It is usually then sufficient to collect 20 fractions, with fraction collection and TLC monitoring being effected simultaneously. When components of the mixture are widely separated, it is appropriate to switch to a more polar eluant after the less polar components have come off the column. The entire process of column construction, elution, and fraction analysis usually takes a little less than 1 h.
We have used a variety of solvent mixtures following this procedure. Ethyl acetate in petroleum ether appears to be the most generally satisfactory. For less polar mixtures, CH2Cl2 in petroleum ether is effective, and for very polar mixtures we use ethyl acetate in CH2Cl2. We have found that if it requires more than 40% ethyl acetate in hexane or less than 5% to give a TLC Rf of 0.4 for the mixture to be separated, it is best to switch to the alternative less polar or more polar solvent system. While it is possible to plot "most effective column eluant" as a function of TLC Rf, derivation of the most effective solvent system for a given separation is still best done empirically.6
Column Diam. |
Silica Gel |
Sample |
Fraction Size |
10.4 mm | 1 g | 0.03 g | 1 mL |
14 mm | 2.5 g | 0.06 g | 2.5 mL |
19 mm | 5 g | 0.1 g | 5 mL |
24 mm | 10 g | 0.2 g | 10 mL |
41 mm | 50 g | 1 g | 50 mL |
60 mm | 100 g | 3 g | 100 mL |
120 mm | 300 g | 8 g | 300 mL |
170 mm | 500 g | 15 g | 500 mL |
Typical column sizes used are shown in Table I. The five smaller columns are packed and run as described in Figure 1. For the four smallest, commercial7 columns are used as received. Air pressure is introduced through a glass tube inserted through a one-hole rubber stopper in the top of the column (Figure 2). It is convenient to maintain column pressure with laboratory compressed air, delivered through a length of Tygon tubing having a small syringe needle inserted in it for a bleed.3 The same procedure is followed for the 50-g column, except that the top of the commercial column is modified by attaching to it a female 35/20 ball joint. The male joint is necked down to a tubing connector for the air line and secured to the female joint with a screw clamp (Figure 2). The three largest columns8 are also packed and run as described, except that aspirator vacuum9 is substituted for air pressure. Fractions are collected in Erlenmeyer flasks by using a vacuum adapter as shown in Figure 3. Again, it is important to close the stopcock at the bottom of the column before releasing the vacuum to change fractions.
The procedure described here, besides using a less costly grade of silica gel, appears to offer substantially better resolution than is claimed for the obvious alternative, flash chromatography.10,11 This is not a minor consideration, even for "one spot" reactions. We have routinely observed6 that samples purified as outlined here, followed by bulb-to-bulb distillation to remove traces of solvent residue, are satisfactory for elemental analysis.
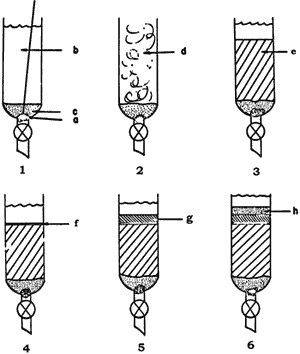
Figure 1.
Column Preparation
Columns used are described in the text.
- A glass wool plug (a) is gently inserted in the bottom of the column and held in place with a wire plunger (b). Column eluant is added, and the glass wool plug is covered with an even layer of sand (c). The sand is gently tapped to remove trapped air bubbles, and the wire plunger is removed.
- The appropriate weight of TLC mesh silica gel is added as a slurry (d) in the column eluant. The stopcock is opened, and the solvent is allowed to drain (5-10 min), partially compacting the column.
- Air pressure (5-15 psig) is applied to the top of the column with the stopcock still open, completing (5 min) column compaction (e). The stopcock is then closed, and the pressure is released. Note that it is essential after the column has been pressurized to always close the stopcock at the bottom of the column before releasing the pressure.
- A circular piece of filter paper, cut to fit the inside of the column, is added. It will slowly settle through the eluant on to the top of the silica gel bed.
- The material to be chromatographed is taken up in an appropriate volatile solvent (CH2Cl2 is usually satisfactory), coarse silica gel5 (10% of TLC mesh silica gel dry weight) is added, and the solvent is removed in vacuo to give a free flowing powder. The use of a glass frit adapter12 between the flask containing the silica gel and the vacuum line is recommended. The eluant is run through the column by the application of air pressure or removed by pipette so that it stands 1-2 cm above the filter paper, and the impregnated coarse silica gel (g) is added.
- A layer of sand (h) is gently added. The eluant is run down to the level of the sand (air pressure), the sides of the column are rinsed down with a little eluant, and that is run (air pressure) down to the level of the sand. The column is gently filled with eluant, air pressure is applied, and fractions are collected.
Figure 2.
Top of Column Assembly
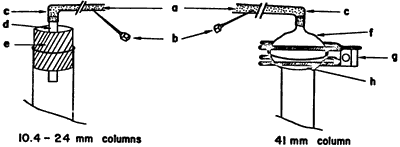
a) Laboratory compressed air. b) Small syringe needle for
bleed. c) Tygon tubing. d) Glass tubing. e) Rubber stopper.
f) Male 35/20 spherical ground glass joint. g) Screw clamp.
h) Female 35/20 spherical ground glass joint.
Figure 3.
Vacuum Adapter for Larger Columns
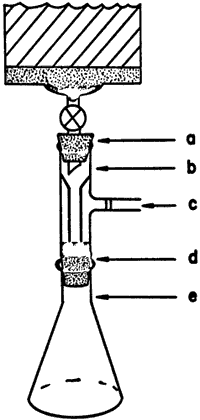
60-170 mm column
- One-hole rubber stopper
- Vacuum adapter, Ace 5260-10
- Aspirator vacuum
- Rubber filter funnel adapter
- Erlenmeyer flask