Abstract
A novel, convenient synthesis of N-(diethoxyphosphoryl)aziridine is described. Application of this compound as a synthetic equivalent of an a2 type synthon for aminoethylation of Grignard reagents is demonstrated.
Although Grignard reagents have been widely used in organic synthesis, their application for the preparation of amines is, compared to other methods, of limited value only. Several primary amines have been obtained in moderate to high yields by reacting 2 moles of alkylmagnesium bromides with 1 mole of methoxyamine.2,3 More recently O-(diphenylphosphinoyl)hydroxylamine has been proposed as a reagent of choice for electrophilic amination of carbanions. The introduction of an amino group via this reagent provides an easy synthetic method for the amination of a variety of Grignard compounds.4,5
Even in those cases when only moderate to fair yields are obtained, the reactions may prove useful due to the easy access and stability of the reagent as well as its low tendency to undergo side reactions. The electrophilic amination of organometallic reagents including Grignard compounds has been recently the subject of a review.6

Scheme 1
Another synthetic approach to primary sec-alkylamines involves the addition of Grignard and organolithium reagents to N-sulfonyl aldimines7 and N-(diphenylphosphinoyl)aldimines8 generated in situ from the appropriate starting materials. All the above mentioned procedures suffer from the disadvantage of using rather expensive reagents and have met with varying degrees of success in the construction of amine molecules. Some other approaches to aminoethylation of various nucleophiles should be also mentioned.9
Scheme 2
In order to circumvent the use of strongly toxic and not commercially available aziridine we have developed a new approach to 3 starting from readily accessible 2-chloroethylamine hydrochloride (1). This salt could be easily phosphorylated in chloroform using the preformed diethyl phosphorochloridate- triethylamine complex (Scheme 1). Crude diethyl N-(2-chloroethyl)phosporamidate (2) formed in high yield (~80%) and spectroscopically pure according to 31P NMR was subjected to cyclization in a solid-liquid two-phase system consisting of benzene and a mixture of solid, powdered sodium hydroxide/potassium carbonate. The reaction proceeded smoothly at room temperature and in the presence of 1 mol% of tetrabutylammonium hydrogen sulfate it was completed (31P NMR) after 4 hours affording N-(diethoxyphoshoryl)aziridine (3) in 64% overall yield. It was found that in the absence of PTC catalyst cyclization is extremely slow, and far from completion after 24 hours, at room temperature.
Table 1.
Aminoethylation of
Organomagnesium Bromides
Product |
R | RMgBr (eqv.) | Yield | mp (°C) (Lit.11 mp) |
5a |
Et | 2 |
90% |
118-120 (119-119.5) |
5b |
Bu | 1.75 | 70% |
125-127 (124-125) |
5c |
t-BuCH2 | 3 |
52%c |
- |
5d |
Me2CH(CH2)2 | 2 | 69% |
107-109 |
5e |
PhCH2CH2 | 1.75 | 56% |
141-142 |
5f |
CH2=CH | 2 | 70% |
103-104 |
5g |
i-Pr | 3 | 75% |
99-101 |
5h |
3-pentyl | 3 |
78%c |
- |
5i |
c-C5H9 | 2 | 72% |
127-129 |
5j |
Ph | 2.5 | 83% |
176-178 (171-172) |
5k |
1-naphthyl | 3a | 81% |
185-187 |
5l |
p-MeO-C6H4 | 3 | 83% |
148-150 |
5m |
t-Bu | 5b | 77% |
257-260 |
5n |
CH2=CH(CH2)2 | 3 | 73% |
111-113 |
Notes:
- 15 mol% of CuI was needed to
ensure complete opening of 3. - 10 mol% of CuI was used.
- Isolated as free amine.
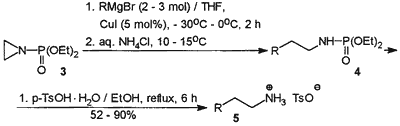
In a search for a versatile, straightforward and relatively inexpensive access to primary amines from Grignard reagents we recently turned our attention to N-(diethoxyphosphoryl)aziridine (3), which can be considered as potential synthetic equivalent of an a2 type +CH2CH2NH2 synthon with the N-protected amino function. This activated aziridine, when subjected to nucleophilic ring opening followed by deprotection, was expected to transform organomagnesium compounds into primary amines having carbon chains elongated by two carbon atoms. N-(Diethoxyphosphoryl)aziridine (3) has been known since 1956, when it was prepared by phosphorylation of aziridine with diethyl phosphorochloridate in the presence of triethylamine.10
Contrary to expectations, N-(diethoxy- phosphoryl)aziridine (3) was totally unreactive towards Grignard reagents in tetrahydrofuran. However, nucleophilic ring opening of 3 by means of 2-3 equivalents of organomagnesium bromides occurred smoothly and cleanly in the presence of 5 mol% of copper(I) iodide at 0°C to give diethyl N-alkyl(aryl)phosphoramidates (4) in high yield and excellent purity (31P NMR, 1H NMR). Crude phosphoramidates 4 (Scheme 2) were dephosphorylated by refluxing with p-toluenesulfonic acid monohydrate in ethanol. Ammonium tosylates 5 were isolated by evaporation of solvent followed by precipitation with diethyl ether and recrystallization from ethanol/diethyl ether if necessary.
Yields, melting points and the relevant spectral assignments of 5 are compiled in Tables 1 and 2. The outlined protocol for the synthesis of 5 represents the first, versatile, and economically attractive approach to one-pot aminoethylation of Grignard reagents providing the corresponding primary amines in high yield and excellent quality without tedious purification.
Experimental
All solvents and reagents were of reagent grade and were purchased from Fluka. The solution of vinylmagnesium bromide in THF was purchased from Aldrich. All bp and mp (determined in open capillaries) are uncorrected.
N-(Diethoxyphosphoryl)aziridine (3)
A partially crystalline mixture of diethyl phosphorochloridate (34.5 g, 0.2 mol) and Et3N (40.4 g, 0.4 mol) was added, at 0°C with efficient stirring and external cooling (ice-salt bath), to the suspension of 2-chloroethylamine hydrochloride (1, 23.2 g, 0.2 mol) in CHCl3 (200 mL). Stirring was continued for 1 h at 0°C. Et3N·HCl was then filtered off and washed with benzene. The solution was washed with cold water (~30 mL), dried (MgSO4), and concentrated in vacuo to give 34.5 g (80%) of crude diethyl N-(2-chloroethyl)phosphoramidate (2) with 98-100% purity by 31P NMR (δ = 8.52).
A mixture of crude 2 (10.8 g, 50 mmol), powdered NaOH (2.0 g), finely powdered K2CO3 (10.0 g), Bu4NHSO4 (0.2 g, ca. 0.5 mmol), and benzene (100 mL) was stirred efficiently at r.t. for 4 h. Solid inorganic salts were filtered off, washed with benzene (50 mL), and the solution was concentrated under reduced pressure. The residue was distilled in vacuo to afford N-(diethoxyphosphoryl)aziridine (3) as a colorless oil; yield: 7.2 g (80%); bp 118°C/12torr; (Lit.10 bp 108.5°C/9.5 torr); nD20 1.4343; (Lit.10 nD20 1.4362).
Aminoethylation of Organomagnesium Bromides; General Procedure for Ammonium Tosylates (5)
CuI (95 mg, 0.5 mmol) was added with stirring to a solution of organomagnesium bromide (10-30 mmol, see Table 1) in THF (30-50 mL, depending upon the solubility of the Grignard reagent) cooled to -30°C (acetone/dry ice bath) and the mixture was stirred for 10 min. A solution of N-(diethoxyphosphoryl)aziridine (3, 1.79 g, 10 mmol) in THF (5 mL) was then added and the cooling bath was removed to allow the temperature of the reacting mixture to rise slowly up to 0°C (occasional cooling with an ice-water bath, if necessary). Stirring was then continued at 0°C for 2 h. The coloration of the mixture changed from yellowish-grey to dark blue. The resultant mixture was quenched with sat. aq NH4Cl (~20 mL) below 15°C (occasional cooling). The aqueous phase was extracted with CH2Cl2 (20 mL) and the combined organic solutions were dried (MgSO4) and concentrated under reduced pressure to give spectroscopically pure 4.
Crude 4 was dissolved in EtOH (10 mL) and refluxed with TsOH·H2O (1.9 g, 10 mmol). The resultant solution was concentrated, diluted with Et2O (20 mL), and refrigerated overnight. Crystalline ammonium tosylate 5 was filtered off, washed with Et2O and recrystallized from EtOH/Et2O. For non-crystallizing ammonium tosylates (5c and 5h) the crude dephosphorylation products were made strongly alkaline with NaOH and steam distilled. The distillate (ca. 100 mL) was saturated with NaCl and the free amine was extracted with CH2Cl2 (3x20 mL). Extracts were dried (MgSO4), evaporated, and distilled bulb-to-bulb to give pure free amines.
Yields, melting points, and spectroscopic data of ammonium tosylates 5 are compiled in Table 1-2.