Formylation of Grignard Reagents using 2-(N-Methyl-N-formyl)-aminopyridineD. Comins & A Meyers [ Synthesis 403-404 (1978) ][ Back to the Chemistry Archive ] Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523, U.S.A. In recent years a number of methods have been reported which convert Grignard reagents to aldehyde precursors and after a subsequent step release the free aldehyde1. We wish to report an efficient one-step reaction which results in direct formylation of a variety of Grignard reagents using the novel reagent,1.
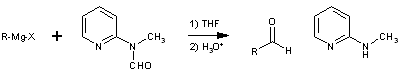 The latter is prepared in large quantities from commercially available 2-aminopyridine in two simpe operations and is regenerated as the N-methyl derivative 3 in equally high yields. The reaction is carried out by simply adding the Grignard reagent to a solution of 1 in tetrahydrofuran at 0° and then quenched after a few minutes in dilute aqueous acid. The aldehydes 2 are obtained in high yield in generally high states of purity. The examples in the Table indicate the effectiveness of this method for aryl, alkyl, vinyl, and acetylenic Grignard reagents. Many of the earlier techniques do not exhibit this general utility. Furthermore, the previous methods invariably lead to an aldehyde in its acetal, aminal or thioacetal form which must be cleaved in a spearate synthetic operation frequentyl causing aldehydic decomposition or other modes of product loss. Although Grignard reagents have been reported to undergo formylation with dimethylformamide2, the presence of the extra ligand (pyridyl nitrogen) and the ready formation of a six-membered chelate 4 prohibits release of the aldehyde under the reaction conditions. This neccessarily protects the aldehydic product from further reaction with Grignard reagent. A similar concept has been described by Mukaiyama3 in the reaction of Grignard reagent with S-(2-pyridyl)-thioates to form ketones.
b Yield of isolated aldehyde of purity > 95% as determined by G.L.C. analysis (conditions: 10% UCW on AW Chrom. W 60-80 mesh, 6' X 1/8" alumina, program 110-200°). I.R. (film): Vmax= 1690cm-1. 1H-N.M.R. (CDCl3): δ=3.35 (s, 3H); 7.12 (m, 2H); 7.74 (d of t, 1H); 8.38 (m,1H); 9.30 ppm (s, 1H). 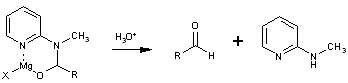
Preparation of 2-(N-Methyl-N-formyl)-aminopyridine(1): The formamide is methylated as follows. A solution of potassium t-butoxide (33.7g, 0.30 mol) in tetrahydrofuran (500 ml) under nitrogen is treated with the formamide from above (29.1g, 0.27 mol) and the mixture stirred at room temperature for 15 min and then 30 min at reflux temperature. The suspension is then cooled to room temperature and methyl iodide (18.7ml, 0.30 mol) is added. The mixture is heated under reflux, with stirring for 18 h, combined clear solution is then evaporated leaving a yellow oil which is distilled to give 1; yield: 26.3g (80%); b.p. 71-72° /0.005 torr.
General Procedure for Formylation of Grignard Reagents:
References:
|