Abstract
A rationale is presented for the investigation of the synthesis and pharmacology of 3-methoxy-4,5-methylenedioxyphenylisopropylamine (MMDA) as a potential psychodysleptic compound; these experiments are reported. The chemical synthesis and physical properties of this compound are described. The pharmacologic effects of MMDA in several animal species are presented, as are its clinical effects in man. The animal pharmacology was generally unremarkable, except for a hypotensive effect in the dog, and the therapeutic index (LD50 rat/MED50 human) was 85. The subjective effects of MMDA in man include an enhancement of feeling and of eyes-closed visual imagery, but no hallucinogenesis or other disturbance of the sensorium. Reality testing and environmental contact are not affected except for a tendency to withdraw into a state of drowsiness, or into a world of fantasy and visual imagery. The induced state of increased availability of emotion was easily manipulated in the psychotherapeutic setting used, and appeared to lead to an enhanced insight into subconscious content.
The psychopharmacologic activity of mescaline (I) has prompted the synthesis and pharmacologic evaluation of a large number of analogs. The structure-activity relationships of these analogs, and the chemical relationship of mescaline to the neurohumoral catecholamines have led to the proposal of some structural relatives as potential endogenous psychotoxins and causative agents in mental illness18. This report describes the chemistry and pharmacology of one of these analogs, 3-methoxy-4,5-methylenedioxyphenylisopropylamine (MMDA).
The first mescaline analog with demonstrated psychotomimetic activity was 3,4,5-trimethoxyphenylisopropylamine (TMA, II). This homolog of mescaline contains the phenyl ring and side chain of the sympathomimetic stimulant, amphetamine. The synthetic details were first described by Hey4 and details of human pharmacology have been reported by Peretz et al.9 and Shulgin et al.12. It was found to produce changes in sensory perception (of color, perspective and time) at half the dosage required of mescaline, although the qualitative nature of the intoxication was somewhat different. Homologs of TMA have been prepared by progressive extension of the alpha-alkyl chain13 but the absence of any quantitative increase in psychotropic potency has led to the conclusion that the amphetamine-like three carbon chain represents an optimum structural feature. The replacement of two of the adjacent methoxyl groups of TMA with the heterocyclic methylenedioxy counterpart, leading to the title compound 3-methoxy-4,5-methylenedioxyphenylisopropylamine (MMDA, III), was a logical step suggested by three separate lines of research.
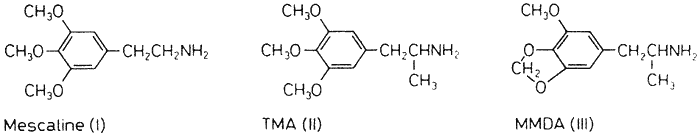
The first suggestion came from the alkaloids found to accompany mescaline in the peyotl cactus Lophophora williamsii. Although many of the tetrahydroisoquinolines present are clearly related to mescaline in that they are trimethoxy derivatives, a number of these isolates have a methylenedioxy bridge in place of adjacent methoxyl groups11. There seems to be a roughly equal representation of these two chemical families (dimethoxy versus methylenedioxy) in the cactus.
A second clue was found in the reports of the psychotropic activity of 3,4-methylenedioxyphenylisopropylamine (MDA) in humans1,8. This compound, although lacking a methoxyl group of the title compound, does have the methylenedioxy ring structure. The analogous dimethoxy counterpart (3,4-dimethoxyphenylisopropylamine) apparently does not produce perceptual alterations or other related subjective phenomena in subjects who did experience such changes with MDA3. It seemed clear that the replacement of two vicinal methoxyl groups with a methylenedioxy ring leads to an increase of potency.
A third argument came from studies of the essential oils. As we have pointed out in a previous report17, there are a number of natural essential oils with various combinations of methoxyl and methylenedioxy substituents on the ring. At the time the work reported here was begun, two compounds (MDA and TMA) with ring structures identical to two of the essential oils (safrole and elemicin) but with a phenylisopropylamino side chain, were known to have psychopharmacologic activity in humans1,9. Since elemicin is readily convertible to TMA in vitro, it seemed a reasonable projection that myristicin might be similarly converted to MMDA. This conversion was successful and this procedure proved to be generally applicable to the essential oils18. A preliminary report by Shulgin14 has described MMDA as being active in humans as a psychotomimetic, and an outline of the synthesis, as well as the supporting animal and human pharmacology, are presented here in detail.
Materials and Methods
Myristicin serves as the most convenient starting material for the synthesis of MMDA. The details of its isomerization to trans-isomyristicin, to myristicinaldehyde, and to the β-nitromyristicin precursor of MMDA have been described16. The lithium aluminium hydride reduction10 of this nitrostyrene, yielded a base easily isolated as the hydrochloride salt, recrystallizable from isopropyl alcohol, mp 190-191°C.
A number of preliminary animal studies were conducted. In mouse experiments, male albinos weighing approximately 25 g were injected either intravenously or intraperitoneally with solutions of MMDA·HCl. Solutions were approximately 1-percent MMDA at the highest level made up in physiological saline. In rats; behavioral and toxicologic data were obtained by oral administration (stomach tube) of MMDA·HCl in a 10% solution in distilled water. Male albino rats (Holtzman strain) of approximately 250 g weight were employed. Respiratory and cardiovascular effects of MMDA were observed in the anaesthetized dog (beagle, average weight 8.6 kg). Phenobarbital was administered intravenously at a dose of 30 mg/kg with supplements necessary to maintain satisfactory anaesthesia. The vagi were sectioned, the tracheae were cannulated, and polyethylene catheters were inserted into the femoral artery and vein. All injections (either 1.3 or 4.3 ml per dose, in physiological saline, and at levels of 0.3, 1, 3, 10, 30 and 100 mg/kg) were given through the venous cannula, and femoral arterial pressure was measured via a Statham transducer. The EKG was recorded using standard limb leads. Respiratory expirations were recorded kymographically with a tambour connected to the tracheal cannula side arm.
The human pharmacology was studied at dosages that did not exceed those required to achieve psychotropic symptoms, the effective levels having been previously determined as being approximately 2 mg/kg15. The doses employed varied between 100 and 350 mg, but most of the subjects received 120-150 mg. MMDA was administered orally, as the hydrochloride salt, to 13 male and 7 female volunteers ranging from 24 to 50 years of age, in a psychiatric clinic setting. Eight of the subjects were at the time receiving therapy for various neurotic complaints, and two more were interested in the experience mainly in terms of the benefit that might be expected from experimentation, during the session, with certain psychotherapeutic procedures. Fifteen of the subjects had had one or more sessions with either LSD or mescaline, either for experimental or for therapeutic purposes. Thus, it was possible to compare the effects of these latter psychotropic materials directly with those of MMDA.
Results
The LD50 in mice was 150 mg/kg i.p. and 55 mg/kg i.v. The minimal effective doses (MED50) were also dependent upon the manner of administration; the MED50 i.p. was 30 mg/kg and the MED50 i.v. was 5 mg/kg. With allowance for the dose differences as defined by the route employed, the qualitative nature of the behavioral changes was quite consistent. At 30 mg/kg i.v. an increase of locomotor activity was almost immediately evident, and persisted for several hours. There was a mild increase in respiratory rate, distinct mydriasis, and an increased sensitivity to sounds. The initial decrease in the sensitivity to touch (noted only at this high level) was replaced by hypersensitivity within an hour, and this lasted for an additional 6 or 8 h. At lower levels, (10 mg/kg i.v.) only this latter hypersensitivity was noted and both the respiratory and locomotor changes were still evident. Similar reactive signs resulted from the i.p. administration of MMDA, but the dosage requirements were several-fold higher.
The LD50 in rats was 170 mg/kg orally. Gross autopsies performed on the rats that died generally showed hemorrhagic lungs, dark liver, and congestion both in the adrenals and the kidneys. Death occurred within the first 3 h following administration. The MED50 of orally administered MMDA was 20 mg/kg. As with the mouse studies above, hyperactivity was noted within 10 min of ingestion of doses representing 100 mg/kg (increased respiration rate, piloerection, and tremors) but these signs were lost completely at 4 h. All rats, including those surviving the acute trials at levels of the LD50, showed normal weight gain during the following 2 weeks, and showed no significant gross pathology at autopsy after this period.
In the dog, no heart-rate changes were noted up to the level that achieved complete cardiac arrest (100 mg/kg) except that at lower levels there were T-wave changes in the EKG, from inverted to upright. Only minimal depressor responses appeared at the lowest doses (1 mg/kg). Maximum hypotensive effects (at 10 mg/kg) decreased the blood pressure from 200/142 to 60/50. Although the rate of respiration was substantially unaltered over the entire dosage spectrum, changes in the character were noted beginning at 1 mg/kg. Whereas in the control periods, inspiration was slow and expiration rapid and forceful, in periods of drug administration, inspiration was prolonged and deep, followed by slow passive expirations. Arterial depressor and respiratory effects were rapid in onset (10-20 sec). The effects of the 30 mg/kg dose indicated tachyphylaxis with a more rapid return to normal of both arterial pressure and abnormal respiration than after the 10 mg/kg dose. The duration of the arterial depressor response at 10 mg/kg was approximately 45 min; and the altered respiration persisted for 10-15 min.
In human subjects, the first symptoms appeared within 30-60 min following administration. Moderate mydriasis was constant, and slight to moderate dizziness was noticed by most of the subjects. Frequent somatic sensations were those of heat and cold, or trembling. The latter corresponded to actual trembling of the arms or lower jaw in five subjects. On one occasion under the effects of 250 mg, a pendular nystagmus was observed in all directions of gaze, and on two occasions a difficulty in focusing was reported. Nausea was present in three subjects during a brief interval, and in one it led to active vomiting.
The psychological effects were mild, so long as the experience was allowed to develop spontaneously. In general, whenever psychotherapeutic techniques were attempted, responses were exaggerated beyond those expected from similar procedures under normal circumstances. Under the effects of MMDA, intense feelings could be aroused, and emotional insight seemed to be facilitated. The spontaneous phenomena most frequently reported were the accentuation of feelings (anxiety, euphoria, loneliness, loving warmth), the visualization of images (with eyes closed), a state of drowsiness and muscular relaxation, and an overestimation of elapsed time. The imagery was generally realistic arid related to everyday perception of people, landscapes, or objects. When no spontaneous imagery was reported, it was generally possible to elicit it by calling to mind some scene (for example, a dream episode). The effects usually reached a peak after the first hour following the initial symptoms, diminished during the second hour, and had disappeared by the end of the fifth hour.
Discussion
The therapeutic index of MMDA (LD50/MED50) varied somewhat; in mice it was 5 i.p. and 11 i.v., and in rats 8.5 orally. In humans, however, undoubtedly due to the greater sensitivity of measurement of the psychoactive dose level, a therapeutic index based on LD50 rat oral/MED50 human oral yields a value of 85, a satisfactory margin of safety.
The rather striking hypotensive effect of MMDA in the dog is worthy of some note. Possibly analogs of MMDA could be synthesized which would be free of psychoactive effects, and which might be useful as hypotensive drugs. This depressor effect is in contrast to the marked pressor action of amphetamine, and indicates that the amphetamine-like isopropylamine side chain in the case of MMDA does not produce the autonomic effects of amphetamine.
This series of pilot clinical trials was not designed for a detailed study of perceptual and emotional effects in human subjects. It does appear that this compound is an effective psychodysleptic drug. It has characteristic effects which were not anticipated and are distinctly different from the effects of mescaline or LSD as observed by other authors and by one of us (C.N.) in many years of clinical experience. A brief discussion of these differences follows.
- The effects were of shorter duration, about 4 h compared to 8-10 h for LSD7 or mescaline2.
- The subjects' experiences were more 'familiar', i.e. closer to the quality of everyday experience. Both the 'transcendental' and 'psychotic' aspects that are frequently described as part of drug experiences with hallucinogens5,2, were here observed only rarely. True depersonalization was not observed at all.
- The content of the eyes-closed imagery was in general realistic, and was only occasionally mythical or 'archetypal' as has often been observed with LSD or mescaline5,2.
- The experience of MMDA intoxication was easy to control by the subject himself, even at the highest dosages employed here. He feels that he has the choice of giving in to a feeling of fantasy or of withdrawing his attention from it; thus, the experience is not spontaneously overwhelming, as it often is with LSD. As a result of this the subject's contact with the environment and his cognitive processes are not disrupted. The subject may feel that he is under the influence of a drug only when his eyes are closed and he is attending to the images available. On other occasions, the subject might feel that the effects have subsided, or in other cases not even yet begun, until he volunteers some expression that reveals his feelings; the experience can then reach a peak of intensity through deliberate concentration and guidance by the therapist.
- Visual phenomena with open eyes, such as enhancement of color or distortion of facial expressions or objects, were observed with MMDA in only three instances, in all three cases at the large dosages. Phenomena such as imagery filling the visual world, illusions or hallucinations frequently reported with LSD5 were not observed here.
- Anxiety has been a more prominent feature with MMDA than with LSD or mescaline. Hollister6, for instance, found unusual tension or anxiety in only 40% of his subjects with LSD or mescaline. Most of the subjects studied here expressed anxiety, but it invariably led the subject to deal with some personal situation by virtue of the therapeutic setting used. Muscular tremor occurred in only 25% of the cases reported here, but it was very intense when it did appear. It could not always be related to subjective anxiety, but was suggestive of some alternate expression of feeling. Hollister6 found 'trembling inside' in 65% of his LSD and mescaline subjects.
MMDA appears to share the properties of mood-intensification, feeling-enhancement and minimal sensory distortion previously described for MDA8 and 4-bromo-2,5-dimethoxyphenylisopropylamine19. We suggest that these compounds represent a new class of psychopharmacologic agents, distinct from the psychotomimetics, which deserve further investigation as useful drugs in treatment of neuroses.
Summary
The pharmacologic effects of MMDA in animals appear to be relatively mild until near-lethal levels are reached, but the therapeutic index (LD50 rat/MED50 human) is large. The psychodysleptic effects in human subjects seem to be concentrated in the areas of feeling enhancement and eyes-closed visual imagery, in marked contrast to the profound distortions of the sensorium found with LSD or mescaline. The differences are sufficiently great that it would be inaccurate to refer to MMDA as a psychotomimetic or hallucinogenic drug. With MMDA, reality testing and environmental contact are not affected except for a tendency to withdraw into a state of slumber or into a world of fantasy. This state of increased availability of feelings has been shown to be easily stimulated and manipulated in a therapeutic setting, where it appears to lead to an enhanced insight into subconscious content.