Synthesis of Nitrazepamby Psycho Chemist2-Amino-5-Nitrobenzophenone2-Amino-5-Nitrobenzophenone, an essential precursor for the synthesis of Nitrazepam, is only offered by Aldrich, the treason-DEA-company, and that for 150 Deutschmarks per 100 g ( 75 US-$ per 100 g) makes it necessary to find a good way to make it. NOTE: The Zinc Chloride mentioned in these syntheses is to be fresh and anhydrous, or it won't work. Old and bad method, but easy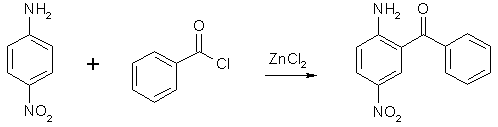
The direct condensation of 138g (1 mole) 4-nitroaniline, 352g (2.5 moles) benzoyl choride and 215 g (1.6 moles) ZnCl2 at 200°C for 2 h (stirring, refluxing, moisture protection), and hydrolysis after cooling down, washing out three times with boiling water (1 L) at 120°C and hydrolysis of the brown-grey residue with 500 mL 75% (V/V) sulfuric acid for 40min. at 140°C (important or product will decompose, concentration, time, temperature), partially neutralizing with ammonia to pH 2 (!) gives a precipate, which consists of 2-amino-5-nitrobenzophenone and benzoic acid. This is stirred with 1 L 20% NaOH for 3 h and suction filtered, then water-washed (2 L) and dried (vacuum, 80°C). The product should melt above 145°C or is crystallized from petroleum ether (+ some benzene). The yield is only 15% (calculated on nitroaniline!), making 36 g! One improvement: before re-cooling of the original condensation mixture, a distillation head is substituting the reflux condenser and slowly vacuum is applied to distill off any unreacted benzoyl chloride. This is reused. It saves material and the washing becomes not a so vigorous process. OUR (now occupied) German Democratic Republic (Eastern Germany) found a better method: A presynthesis (95% yield) is necessary: N-benzoyl-4-nitroanilineDissolve 138 g (1 mole) 4-nitroaniline in 1 l dry pyridine and add dropwise with ice-coling - internal temp below 20°C - 141 g (1.01 mole) benzoyl chloride over 2 h. Stir at 30°C 24 h and evaporate to dryness at the rotovap in vacuo. Recover the expensive dist-off pyridine! The rest is suspended in 1 L 40°C water and the product filtered and water-washed to remove pyridine rests. The mass is suspended in a 2 L flask in 500 mL toluene, a water-trap and a reflux condenser are attached and the mixture refluxed to remove any water, then the toluene is distilled off in vacuo (recover) and then, it is dried at 80°C in vacuo and weighed (weigh the flask before and after). The yield is about 242 g N-benzoyl-4-nitroaniline. 2-amino-5-nitro-benzophenone - DDR (GDR) patent 74798Now example 1: In the 2l-flask, mix 242 g N-benzoyl-4-nitroaniline, 280 mL benzoyl chloride and 200 g fresh dry zinc chloride. Add a reflux condenser and stirr under moisture protection at 170°C internal temp (exact 165-175°C) for 9 h while HCl gas is evolved. My improvement: Then change the condenser by a distillation head and slowly vacuum is applied to distill off any unreacted benzoyl chloride. This is reused. It saves material and the washing becomes not a so vigorous process. Back to the patent: After cooling down to 120°C, 1 l water is added dropwise (vigorous reaction), with stirring, and at 60°C, the granular mass is suction filtered and washed, succed dry. 286 mL conc. sulfuric acid is added to 150 mL water and the dry product (N-benzoyl-2-amino-5-nitro-benzophenone) and the whole is stirred for 40 min. at 140-150°C (do not change). Immediately, it is poured in 1/2 l water. After cooling to RT, the precipitate is suction-filtered and water-washed, then the crude product is stirred with 750 mL 10% sodium hydroxide for 2 h. (removes benzoic acid) Then, the purified product is suction-filtered, water-washed, succed dry and dried at 80°C in vacuum. Yield 90 g, that is 37%. mp 154-160°C, 92-95% pure. Now example 2: Work as in example 1 until you have water-washed the intermediate (the granules of N-benzoyl-2-amino- 5-nitro-benzo-phenone). 286 mL conc. sulfuric acid and 180 mL water are added and the whole is stirred at 120°C (!!) for 4 h and immediately poured into 750 ml water. After cooling to RT, the precipitate is filtered with suction and water-washed, then the crude product is stirred with 750 mL 10 % sodium hydroxide for 2 h. (removes benzoic acid) Then, the purified product is suction-filtered, water-washed, succed dry and dried at 80°C in vacuum. Yield 84 g, that is 35 %. Now example 3: In the 2l-flask, mix 242 g N-benzoyl-4-nitroaniline, 280 mL benzoyl chloride and 200 g fresh dry zinc chloride. Add a reflux condenser and stir under moisture protection at 170°C internal temp (exact 165-175°c) for 9 h while HCl gas is evolved. My improvement: Then change the condenser by a distillation head and slowly vacuum is applied to distill off any unreacted benzoyl chloride. This is reused. It saves material and the washing becomes not a so vigorous process. Back to the patent: After cooling down to 120 °C, 1 l water is added dropwise (vigorous reaction), with stirring, and at 60 °C, the intermediate N-benzoyl-2-amino-5-nitro-benzophenone is vacuum-filtered, water-washed and sucked dry. Mix 160 g sodium hydroxide, 600 mL methanol and 600 mL water and add to the granulated intermediate. This is stirred and refluxed 6 h and cooled to RT. The crude 2-amino-5-nitro-benzophenone is succed off, and water-washed, then stirred with 1 L 8% HCl-solution to remove 4-nitroaniline. The crude product is then suction-filtered, water-washed, succed dry and vacuum-dried at 80°C. Yield 95 g, mp 145-155°C. NOTE: 75% H2SO4 decomposes the benzophenone at temp >130°C when heated longer than 40 min. Nitrazepam (Mogadon, 7-nitro-5-phenyl-1,3-dihydrobenzo-[1,4]-diazepin-2-one)Dissolve 0.206 moles (50 gr.) 2-amino-5-nitrobenzophenone in 250 mL dry chloroform, add over 5 min. 24 mL (314 mmol) chloroacetyl chloride, stir 2 hrs at 35 °C with moisture protection. Evaporate all volatiles at the rotary evaporator, add more 100 mL chloroform to the residue and evaporate again to total dryness. Take up the resultant 2-(chloroacetamido)-5-nitrobenzophenone in 200 mL THF, add 42 g (0.3 moles) Hexamine (hexamethylenetetramine), 900 mL 96% alcohol, and 220 mL (2.95 moles NH3) 25% ammonia solution, density 0.91 kg/L. Reflux for 6 hrs, evaporate (rest in vacuo at the rotary evaporator) all volatiles, add 240 mL toluene and 1 gr. toluenesulfonic acid hydrate and shake. Reflux with Dean-Stark adapter/condenser for 90 min., add 150 mL hot water and let cool down over night. Filter of the brown solid, wash twice with 100 mL water and dissolve in 400 mL dichloromethane. Pack a 30 mm diameter column 5 cm high with silica gel (F254, Merck) / dichloromethane, filter the solution of the crude product through it. Wash the column with 100 mL more dichloromethane (the absorptive filtration will remove some tar), and evaporate the complete filtrate to complete dryness. About 40 grs. of nitrazepam will result. Nitrazepam is a strong tranquilizer and a hypnotic, dosage: 5 mg. 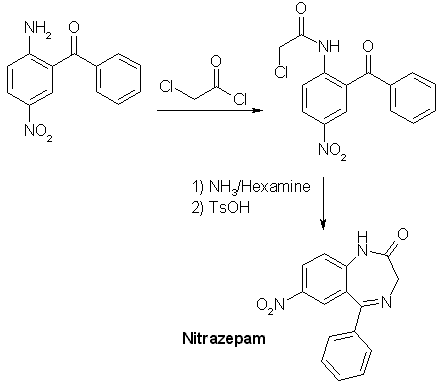 |