Abstract
Reaction of 6-nitro-Δ5-steroids and nitroalkenes in tetrahydrofuran with CdCl2–Mg–H2O furnished 6-ketosteroids and ketocompounds, respectively, in good yield.
Nitro compounds are important precursors in organic synthesis,1-3 the reduction of conjugated nitroalkenes to ketones being one important transformation, and various reagents have been used.4-9 Expensive reagents and stringent reaction conditions are the major drawbacks of most of these reported procedures.
The reaction of SnCl2·2H2O with 6-nitro-Δ5-steroids has been reported to give 5α-hydroxy-6-keto steroids10 and the Nernst potential difference of Cd/Cd2+, Mg/Mg2+ is higher than that of Sn/Sn4+,11 it was therefore expected that the combination of CdCl2–Mg in a protic solvent would be a useful reducing system for the transformation of conjugated nitroalkenes to other functionalities. Our surmise indeed proved to be correct when we treated 3β-acetoxy-6-nitro-cholest-5-ene in tetrahydrofuran (THF) with CdCl2–Mg–H2O system at room temp., within 15 min 3β-acetoxy-5α-cholestan-6-one was obtained in 85% yield. In this reaction 15% of 3β-acetoxy-5β-cholestan-6-one was also obtained.
Table 1
Reduction of nitroalkenes to ketocompoundsa
Entry |
Substrate | Productb | Yieldc | Time |
1 |
3β-Acetoxy-6-nitrocholest-5-ene | 3β-Acetoxycholestan-6-one | 100% | 15 min |
2 |
3β-Acetoxy-6-nitrostigmast-5-ene | 3β-Acetoxystigmastan-6-one | 90% | 15 min |
3 |
6-Nitrocholest-5-ene | Cholestan-6-one | 95% | 15 min |
4 |
3β-Chloro-6-nitrocholest-5-ene | 3β-Chlorocholestan-6-one | 90% | 15 min |
5 |
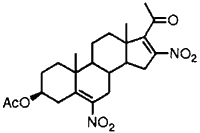 | 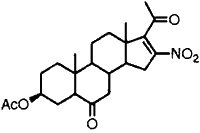 | 90% | 15 min |
6 |
3-Nitrocholest-2-ene | Cholestan-3-one | 95% | 15 min |
7 |
1-Nitrocyclohex-1-ene | Cyclohexanone | 85% | 15 min |
8 |
1-Phenyl-2-Nitropropene | 1-Phenyl-2-Propanone | 80% | 15 min |
9 |
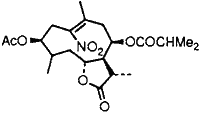 | 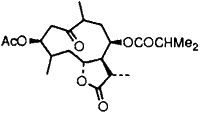 |
60%d |
15 min |
Notes
- ln a typical reaction, 1 mmol of the nitroalkenes in dry THF (3 cm3) was added with stirring to a mixture of anhydrous CdCl2 (8 mmol). Mg powder (15 mmol). Then water (100 mmol) was added dropwise to this mixture over a period of 5 min. The exothermic reaction took place instantly with the liberation of hydrogen. After 15 min, the reaction mixture was thoroughly washed with CH2Cl2 (200 cm3). The CH2Cl2 solution was dried (Na2SO4). evaporated under reduced pressure to give a residue which on purification by TLC gave the product as a gum or solid. All products were characterized by spectral analysis and direct comparison with authentic compounds.
- For entries 1-5, both 5α-ketosteroid and 5β-ketosteroids are formed in a ratio of 85:15, respectively.
- The reaction was conducted with other protic solvents such as MeOH. EtOH. glycols, diglyme and in each case the yield was found to be same.
- ln this reaction 37% of the unreacted starting material was also recovered from the reaction mixture.
In order to assess the scope of the reaction, 1-nitro-1-cyclohexene, β-methyl-β-nitro-styrene and the compound obtained from natural products12 (Entry 9) were treated with the CdCl2–Mg-H2O system and in each case corresponding ketocompounds were obtained in 60–85% yield (Table 1). Although the mechanism of this reduction process is not clearly defined at this stage, the following observations were noted. Anhydrous CdCl2 and Mg in anhydrous THF does not react even after long exposure. But addition of a few drops of water to this mixture initiates vigorous exothermic reaction with evolution of hydrogen (deuterium when D2O is added) and the formation of metallic cadmium particles. Since CdCl2 is not hydrolysed readily and the system has pH ~7, hydrogen is released from the water present and is possibly replaced by magnesium.