In this investigation, a number of aromatic nitro compounds were reduced by the use of activated iron. The method employed was essentially that of Jenkins, McCullough, and Booth1, which had been used successfully for the reduction of 2- and 4-nitrobiphenyl. The method was found to be desirable because the materials are cheap and undesired chlorinated products, which are obtained by certain other methods, are not formed by side reactions.
The purpose of this study was to investigate further the applicability of this method to the reduction of nitro compounds of various representative types.
Experimental
Preparation of Activated Iron
Ten milliliters of concentrated hydrochloric acid was added slowly with stirring to 50g of granulated iron (40 mesh); care was taken to prevent the generation of excessive heat during the course of the ensuing reaction. The activated iron was stirred frequently to prevent the formation of lumps and was allowed to dry thoroughly.
General Procedure
Five grams of the nitro compound was dissolved in 200 ml. of benzene in a three-necked flask which was provided with a reflux condenser and an efficient stirrer. The benzene solution was heated almost to boiling on a steam-bath before the iron was added. Then the activated iron was introduced, and vigorous stirring and refluxing were maintained throughout the course of the subsequent steps. After one-half hour of refluxing, 1 ml of water was added to the reaction mixture; thereafter, small quantities of water were introduced from time to time at such a rate that, at the end of seven hours, 20 ml. of water had been used. Finally, refluxing was continued for an additional hour.
Recovery of Products2
For the isolation of the reduction products, different procedures were found to be necessary:
- In those preparations in which the product was soluble in benzene and was isolated as the hydrochloride, the reaction mixture was freed of iron by filtration, and dry hydrogen chloride was passed through the benzene solution; the insoluble hydrochloride precipitated and was collected by filtration.
- In other instances where the amine which was formed in the course of the reduction was soluble in benzene, the reaction mixture was filtered while still hot, and the iron residue was extracted three times with hot benzene. The extracts were combined with the original filtrate. The crude free amine was obtained by removal of the benzene by distillation, and purification was effected by recrystallization from a suitable solvent.
- With one amine which was insoluble in benzene, the solvent was removed from the reaction mixture by distillation from a steam-bath, the desired product was extracted from the residue with hot methanol, and the iron compounds which were dissolved by the hot solvent were removed by precipitation with hydrogen sulfide. Purification was accomplished by recrystallization.
Identifications of Products
Identifications of the reaction products were made by means of melting points of: (a) the free amines, (b) the acetyl derivatives, or (c) the hydrochlorides.
Results
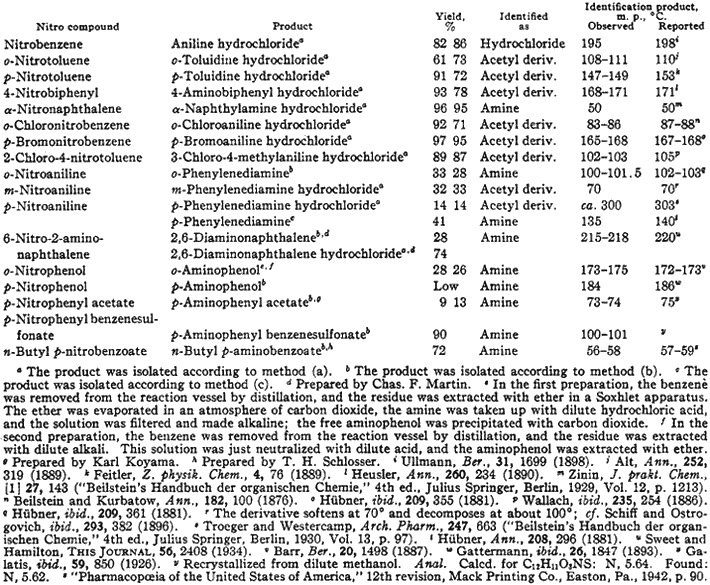
The results which were obtained when various nitro compounds - a number of them bifunctional in character-were reduced by the method outlined above are summarized in Table I.
Table I
Reduction of Aromatic Nitro Compounds
Summary
Seventeen nitro compounds were reduced by the use of activated iron, and products were obtained, for the most part, in good yields. In contrast to methods involving more severe conditions, there was no evidence of undesired introduction into or removal from the amines of halogen. Preliminary results suggested the possible use of this method for the reduction of esters of nitro substituted acids and similar compounds which would be subject to hydrolysis if reduced in acidic or basic aqueous media; this end was realized in several cases.