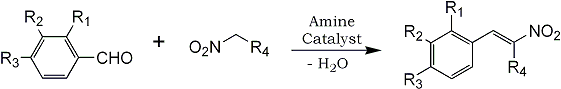
The condensation of nitroalkanes with benzaldehydes to give β-nitrostyrenes has usually been catalyzed by bases such as alcoholic potassium hydroxide1 or alcoholic methylamine2 although the reaction was first carried out using an acid catalyst, zinc chloride3. Ammonium acetate in acetic acid has been used to bring about this reaction in a few instances4-6. Comparisons of various condensing agents have been made for individual compounds7 but no evaluation of the various methods to ascertain the most generally useful procedure seems to have been made. In connection with a project requiring the preparation of various hydroxy and alkoxy substituted 2-phenylethylamines it became necessary to develop a general procedure which could be used with all combinations of nitroalkane and substituted benzaldehyde.
Substituents |
Yielda
|
|||||
R1 |
R2 |
R3 |
R4 |
mp (°C) | Method Ad | Method B |
H |
H |
H |
H |
58-59 |
55% |
60% |
H |
H |
OH |
H |
168-169 |
20% |
60% |
CH3O |
H |
H |
H |
48-49 |
50% |
82% |
H |
H |
CH3O |
H |
86-87 |
40% |
65% |
C2H5O |
H |
H |
H |
37-38 |
30% |
20% |
CH3O |
CH3O |
H |
H |
84-85 |
50% |
60% |
H |
CH3O |
CH3O |
H |
141-142 |
80% |
70% |
H |
CH3O |
OH |
H |
167-168 |
95% |
50% |
H |
C2H5O |
OH |
H |
170-171 |
75% |
78% |
OH |
CH3O |
H |
H |
122-124 |
45% |
75% |
H |
H |
H |
CH3 |
64-65 |
52% |
55% |
H |
H |
OH |
CH3 |
124-125 |
none |
35% |
H |
H |
CH3O |
CH3 |
44-45 |
60% |
80% |
H |
CH3O |
CH3O |
CH3 |
67-68 |
30% |
35% |
CH3O |
CH3O |
H |
CH3 |
78-79 |
50% |
75% |
H |
CH3O |
OH |
CH3 |
100-101 |
trace |
20% |
H |
C2H5O |
OH |
CH3 |
108-109 |
trace |
50% |
OH |
CH3O |
H |
CH3 |
104-105 |
trace |
45% |
CH3O |
CH3O |
H |
C2H5 |
64-65 |
none |
60% |
H |
CH3O |
CH3O |
C2H5 |
78-79 |
none |
52% |
H |
CH3O |
OH |
C2H5 |
79-80 |
none |
40% |
CH3O |
H |
H |
C2H5 |
oilb |
none |
34% |
H |
CH3O |
H |
C2H5 |
oilc |
||
C2H5O |
H |
H |
C2H5 |
oilc |
||
Notes:
a) In most cases the yield is averaged over several runs.
b) Purified by molecular distillation.
c) Not purified
d) Method A: Methylamine Catalyst;
Method B: Ammonium Acetate-Acetic Acid Catalyst.
It was found that alcoholic potassium hydroxide gave generally poor results with most substituted benzaldehydes in the reaction with nitromethane and was completely unsuccessful with nitroethane and 1-nitropropane. Methanolic methylamine was more successful but gave good yields only if the resulting β-nitrostyrene was high-melting and sufficiently insoluble in the reaction medium to precipitate almost as fast as it was formed. With this catalyst, unless the product was removed within a short time after completion of the reaction, high-melting products were formed. These appeared, from molecular weight determination and elementary analysis, to be trimers of the β-nitrostyrene but their structure was not determined. They appear to be similar to the high-melting compound reported by Jansen8 as resulting from the reaction of anisaldehyde with nitromethane. The same compounds were formed when a pure β-nitrostyrene was allowed to stand for a few hours in contact with methanolic methylamine. In order to obtain the maximum yield of nitrostyrene yet avoid the formation of the polymer it was necessary to run a series of experiments with a given aldehyde-nitroalkane combination to find the optimum time. This was a distinct disadvantage in preparing nitrostyrenes from difficultly-prepared aldehydes.
In reactions of benzaldehydes with nitroethane and 1-nitropropane the resulting nitrostyrenes were generally quite soluble in the reaction medium and in these reactions the yield was usually low under the best of conditions.
The most satisfactory general procedure was found to be the use of ammonium acetate in glacial acetic acid. Although in a few cases the yield of nitrostyrene was lower than with methylamine in most cases it was substantially better. The time required for the reaction was much less and no unwanted higher condensation products were obtained even on long heating. The same general procedure was successful for many combinations of aldehyde and nitroalkane. Particularly in reactions involving nitroethane and 1-nitropropane was the superiority of this method evident.
Experimental
The results obtained in the reaction of various substituted benzaldehydes with nitroalkanes using methylamine and ammonium acetate-acetic acid are summarized in Table I.
Methylamine-catalyzed condensations
0.1 mole of the aldehyde and 0.15 mole of the nitroalkane were added to sufficient methanol to just dissolve the aldehyde and to the resulting solution was added 5 ml of 5% methanolic methylamine. When the precipitation of solid appeared to be complete the mixture was chilled and the product was collected. The time required for optimum yield varied from six hours to three days for the reaction of aldehydes with nitromethane and from three days to a week for nitroethane. No relationship between the structure of the aldehyde and the reaction time was observed. Heating the solution increased the amount of high-melting polymer considerably and similarly reduced the yield of desired product. The nitrostyrenes were purified by recrystallization from methanol or dilute acetic acid.
Higher condensation products. In most of the above reactions the nitrostyrene was accompanied by a less soluble high-melting compound. This material was the exclusive product if the reaction was allowed to continue long enough or was produced in high yield if the nitrostyrene was allowed to stand with methanolic methylamine for several weeks. Most of the high-melting products were insoluble in all organic solvents, had very wide melting or decomposition ranges, and appeared to be mixtures. Only one of them, that from benzaldehyde and nitromethane, could be obtained in a pure crystalline form by recrystallization from pyridine. This material was a pale pink crystalline solid, mp 216-218°C.
Condensations with ammonium acetate in acetic acid
The aldehyde (5g), 5 ml. of the nitroalkane, and 2g of ammonium acetate were added to 20 ml of glacial acetic acid. The resulting solution was refluxed for two hours and then poured into ice-water. If a solid product was obtained it was collected and recrystallized from methanol, ethanol, or acetic acid. If the product was an oil it was separated and crystallized, if possible, from one of the above solvents. With some methoxy- and ethoxy-benzaldehydes and 1-nitropropane, as noted in the Table, oils were obtained which could not be made to crystallize and which could not be distilled. One of these, that from anisaldehyde, was successfully distilled using a molecular still to give an oil having the correct analysis for the expected product. It is believed that the same procedure could be applied to the other non-crystalline products.
Summary
A comparison has been made of the effectiveness of methylamine and of ammonium acetate in acetic acid in catalyzing the reaction of, substituted benzaldehydes with nitroalkanes to give β-nitrostyrenes. Ammonium acetate in acetic acid proved more generally useful, particularly in reactions with nitroethane and 1-nitropropane.