Introduction
The conversion of a benzaldehyde into a phenyl-2-propanone is a very important reaction in amphetamine chemistry, and this is one of the less known ways of doing this. It involves many steps and unusual reagents compared to other methods, but it may be of use in those instances where other methods fail for one reason or another. One of the most interesting steps is the last one, where a phenylacetic acid is concerted directly to a phenyl-2-propanone with MeLi. The reaction should be general for all benzaldehydes and phenylacetic acids (except those with a halogen on the ring, which may react with the methyllithium).
In the first step, the benzaldehyde is condensed with hippuric acid (glycine benzamide) with acetic anhydride as a dehydrating agent to form a so-called azlactone, which is then hydrolyzed with NaOH and oxidized by alkaline H2O2 to form a mixture of phenylacetic and benzoic acid. To separate these acids, their methyl esters are prepared, and then the less soluble phenylacetic ester is precipitated by concentration and cooling of the reaction mixture. The methyl benzoate stays in solution. It may be possible to separate the acids by vacuum distillation instead, but I don't know if that would lead to any better yields. Finally, the ester is hydrolyzed and reacted with methyllithium to give the desired phenyl-2-propanone.
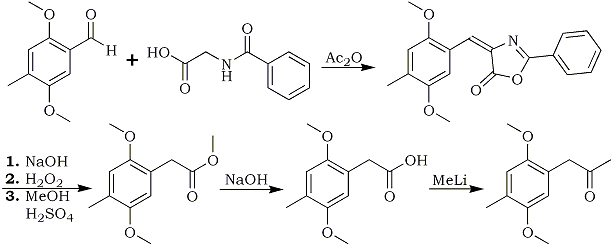
The reaction sequence below is exemplified for the synthesis of the DOM (STP) precursor 2,5-Dimethoxy- 4-methyl-phenyl-2-propanone from 2,5-Dimethoxy-4-methylbenzaldehyde
Experimental
4-(2,5-Dimethoxy-4-methylbenzylidene)-2-phenyl-2-oxazolidin-5-one1
A mixture of 2,5-Dimethoxy-4-methylbenzaldehyde (15.0g), hippuric acid (15.0g), acetic anhydride (26.0g) and anhydrous sodium acetate (7.0g) was heated with stirring at 100°C for 2 hours, cooled and diluted with 100ml ethanol. An orange-red precipitate of the title compound precipitated, yield 23.6g, mp 208-210°C.
Methyl 2,5-Dimethoxy-4-methyl-phenylacetate1
A solution of 4-(2,5-Dimethoxy-4-methylbenzylidene)-2-phenyl-2-oxazolidin-5-one (15.0g) in 100ml 10% NaOH solution was heated under reflux for 12h. The reaction mixture was cooled to 0°C and diluted with 10ml ice-cold 40% NaOH solution. To this solution was added with stirring, a 15% aqueous solution of hydrogen peroxide at such a rate to maintain the temperature below 15°C. After this addition was complete, the solution was left at room temperature for 12h, then acidified with 50ml concentrated HCl and extracted thorougly with benzene [or other non-polar solvent]. The organic extracts was dried over MgSO4, filtered, and evaporated to give a solid (15.5g) which was dissolved in 100ml methanol containing 2ml H2SO4. This solution was heated under reflux for 5h, concentrated and cooled in the fridge, whereupon 9.2g of the title ester precipitated, with a mp of 66-67°C after recrystallization from ethanol
2,5-Dimethoxy-4-methyl-phenylacetic acid1
A suspension of 8g methyl 2,5-Dimethoxy-4-methyl-phenylacetate in 50ml 10% NaOH solution was heated under reflux for one hour, cooled and acidified with concentrated HCl. This gave a precipitate of 2,5-Dimethoxy-4-methyl-phenylacetic acid (7.6g), which after recrystallization from ethanol had a mp of 128-129°C
2,5-Dimethoxy-4-methyl-phenyl-2-propanone1
A solution of 2,5-Dimethoxy-4-methyl-phenylacetic acid (9.0g) in 100ml ether was added slowly to 60ml of a 2.1 M solution of methyllithium in ether. The mixture was heated under reflux for one hour and then added to 200ml ice-water saturated with ammonium chloride. The organic layer was collected and the aqueous portion was extracted with 2x100ml ether. Evaporation of the combined oganic phases yielded the ketone, 4.7g (mp 56-58°C). Acidification of the aqueous phase caused the precipitation of the phenylacetic acid (2.1g).
Preparation of Methyllithium2
Methyliodide (425.7 g, 3.00 moles) was added with stirring to 48 g (7.0 moles) of lithium in 2.5 L of ether under nitrogen at a rate adequate to maintain gentle reflux of the ether. After 24 hours the solution of methyllithium was decanted into a storage vessel filled with nitrogen. The concentration was estimated in the usual way by hydrolysis of an aliquot and titration with 0.1N hydrochloric acid.
Very pure methyllithium can also be prepared from methyl chloride and Li powder containing 1% Na3.