Abstract
A number of published syntheses for the manufacture of controlled substances appear to be impractical for the average clandestine laboratory. A closer inspection of these syntheses may reveal modifications which greatly simplify their application. An excellent example of this is the preparation of phenyl-2-propanone (P-2-P) from allylbenzene. In the prototype published method, oxygen is introduced into the reaction vessel by using a tank of compressed oxygen with a balloon for a gas reservoir. In our modification, oxidation is accomplished with a 30% hydrogen peroxide solution. P-2-P has been prepared by both methods and a comparison made of the reaction mixtures at various times during their synthesis. Additionally, propenylbenzene, a by-product of these reactions, can be converted to P-2-P by modification of a second synthesis.
Background
The investigation of clandestine drug manufacturing laboratories represents a combined effort between the criminal investigator and the forensic chemist. At an early point in an investigation the special agent will frequently request a list of the chemicals and synthesis methods used to produce a controlled substance. Providing these lists is often a very simple assignment the forensic chemist. A general understanding of various chemicals reactions and techniques is a part of the forensic chemist's training, academic background, and experience. Additionally, numerous specific and detailed drug syntheses are also available to him from the open literature. The chemist may, none the less, encounter problems when reviewing published procedures. If the literature procedures do not explicitly illustrate the synthesis of the desired pound, the chemist may erroneously assume that it is not applicable to the clandestine laboratory. This conclusion may, in part, be due to the complicated nature of the procedure or to the apparent requirement for specialized equipment. It may also arise from the failure of the chemist to visualize an application of the literature to the synthesis of the clandestine drug. In this context, the synthesis methods are themselves clandestine; they are "hidden" within the literature. A determined study of literature procedures, however, often reveals that while they do not detail the synthesis of the drug in question, they can be modified to give useful or simple methods for its manufacture. Sometimes this requires only the substitution of appropriate chemicals or certain changes in reaction parameters or catalysts.
Examples of this conceptual approach can be shown by the synthesis of the nonpsychoactive controlled substance phenyl-2-propanone (P-2-P). Halting the clandestine manufacture of P-2-P is of particular interest to enforcement personnel since it serves as the primary precursor in a number of syntheses for amphetamine and methamphetamine. By substitution of the chemicals and through slight changes in procedure, two published syntheses have been modified for P-2-P manufacture. These simple changes are illustrated below and are of the type to be expected of a clandestine drug chemist. By procuring chemicals and using procedures not generally recognized for the production of the controlled substance, the clandestine chemist may improve his chances to escape detection. Each of the two procedures investigated give fair to excellent yields of P-2-P, and, by using the procedures consecutively, yields are greatly increased.
Experimental
Allylbenzene Procedure
The following procedure, which Tsuji et. al.1 used for the preparation of 1-decanone, required only the substitution of allylbenzene (1-phenyl-2-propene) for 1- decene. A three-neck round bottomed flask was fitted with a magnetic stirrer and a pressure-equalized dropping funnel containing allylbenzene.
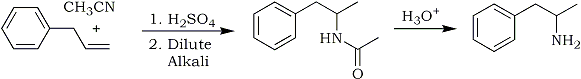
Fig 1:
Synthetic procedure for Phenyl-2-propanone - allylbenzene method
The flask was charged with a mixture of palladium chloride, cuprous chloride, and aqueous N,N-dimethylformamide (DMF). With all outlets securely stoppered and wired down, an oxygen-filled balloon was placed over one neck and the flask contents stirred at room temperature to allow oxygen uptake. After a period of oxygenation, allylbenzene was added dropwise. The solution was continuously stirred under the pressurized balloon. During this period of addition, the color of the solution turned from green to black and gradually returned to green as the reaction approached completion. The mixture was poured into cold hydrochloric acid and extracted with methylene chloride (CH2Cl2). The extract was washed with saturated sodium bicarbonate and dried over anhydrous sodium sulfate. Through filtration and distillation, phenyl-2-propanone and trans-beta-methylstyrene (1-phenyl-1-propene) were recovered. The reaction is shown in Fig. 1. Yields approximate those listed in the "modified" procedure given below.
Modified Allylbenzene Procedure
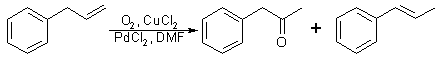
Fig 2:
Synthetic procedure for P-2-P - Modified allylbenzene method
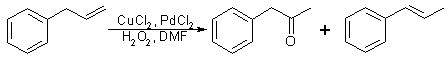
The procedure employed was a simple modification of the preceding reaction. The equipment modification eliminates the oxygen filled balloon, which requires an outside source of oxygen, such as a compressed oxygen tank, and replaces it with a 30% hydrogen peroxide solution. The aqueous DMF solution is thus prepared by using 30% hydrogen peroxide in place of water. The quantity of palladium chloride catalyst was also decreased. This reaction is shown in Fig. 2. The synthesized P-2-P (49% yield) was separated from the reaction mixture by shaking with saturated aqueous sodium bisulfite solution and cooling the resultant mixture. Vacuum filtration of the mixture yielded crystalline P-2-P bisulfite addition product. The two-phase filtrate was then separated and the organic layer containing the trans-beta-methylstyrene (45% yield) was recovered by extraction with CH2Cl2. The recovered trans-beta-methylstyrene still contained several grams of P-2-P and trace amounts of allylbenzene and cis-beta-methylstyrene.
Trans-Beta-Methylstyrene Procedure
Fig 3:
Synthetic procedure for P-2-P - trans-beta-methylstyrene method
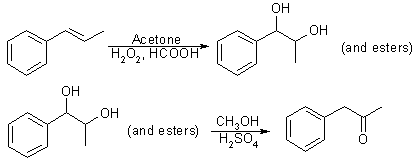
By using a modification of Fujisawa and Deguchi's2 synthesis of 3,4-methylenedioxybenzyl methyl ketone, the trans-beta-methylstyrene by-product of the previous reaction was also converted to P-2-P. This conversion was accomplished by dropping an acetone/trans-beta-methylstyrene solution into a stirred solution of hydrogen peroxide in formic acid2,3. The acidic solution was refluxed and, then, neutralized and extracted with CH2Cl2. After Evaporating most of the CH2Cl2, the extracted mixture of glycol and glycol esters was stirred stirred and heated in a solution of methanol and dilute sulfuric acid. The resulting solution was neutralized with aqueous base and extracted with CH2Cl2 to yield phenyl-2-propanone (93%). The sequence shown in Fig. 3 was adapted from Fujisawa and Deguchi.
Discussion
The synthesis of P-2-P from trans-beta-methylstyrene was performed using the recovered impure side product from the allylbenzene. Commercially obtained pure trans-beta-methylstyrene is also suitable; however, its current cost is approximately four times greater than allylbenzene. The analysis of the reaction mixture near the end of the performic acid oxidation5 suggests the formation of four new components. This type of oxidation primarily yields the glycol and glycol esters.
The mass spectrum of the major oxidation component indicates that it is phenyl-1-hydroxyl-2-formyl-propane. Its isomer, phenyl-1-formyl-2-hydroxy-propane, is also formed. The diformyl glycol and the free glycol, phenyl-1,2-dihydroxypropane is also found in smaller amounts. Hydrolyzing these oxidation products by refluxing with sulfuric acid and methanol produces P-2-P in high yield. The NMR spectrum of a hydrolysis sample removed after 2 h shows P-2-P to already be a major component. After 3.5 h of hydrolysis, virtually the entire sample converted to P-2-P.
Conclusion
Although phenyl-2-propanone is not a sympathomimetic substance in and of itself, it is frequently synthesized in clandestine laboratories to produce the essential chemical for the manufacture of amphetamine or methamphetamine. By modification and coupling of available literature procedures, a sequence is presented for the manufacture of P-2-P. This sequence uses chemicals not normally associated with the clandestine synthesis of P-2-P. Evaluation of these syntheses underscores the diligence required of the forensic chemist to provide enforcement personnel with accurate information. The investigated reactions and procedures are easily performed and, when used consecutively, result in an approximate total yield for P-2-P of 79%.