Abstract
Efficient synthesis of tryptamine by the decarboxylation of tryptophan is described.
Carbonyl Cpd. (2) |
Mole 2/1 |
Time | Yield (g):(%) | |
| R1 | R2 | |||
| none | none | 0 |
6 h | 2.0:12.5 |
| H | (CH2)2CH3 | 0.18 |
9 h | 10.0:62.5 |
| CH3 | CH3 | 0.08 |
3 h | 12.0:75.0 |
| CH3 | C2H5 | 0.07 |
3.5 h | 13,5:84.4 |
| CH3 | (CH2)2CH3 | 0.06 |
2.5 h | 13.8:86.2 |
| CH3 | C(CH3)3 | 0.05 |
6.5 h | 12.6:78.8 |
| CH3 | CH2COCH3 | 0.08 |
2 h | 11.0:68.7 |
| CH3 | (CH2)2CH3 | 0.13 |
6 h | 8.2:51.2 |
| C2H5 | C2H5 | 0.06 |
4 h | 13.6:85.0 |
| CH2-(CH2)3-CH2 | 0.10 |
2 h | 12.0:75.0 | |
| H | C6H5 | 0.15 |
4 h | 9.1:56.9 |
| H | C6H4OH-(o) | 0.07 |
2 h | 10.7:66.9 |
| H | 2-furyl | 0.18 |
4 h | 9.3:58.1 |
| H | 2-pyridyl | 0.02 |
4 h | 12.0:75.0 |
| H | 3-pyridyl | 0.20 |
8 h | 8.0:50.0 |
| CH3 | C6H5 | 0.10 |
2.5 h | 11.0:68.7 |
| C6H5 | C6H5 | 0.20 |
12 h | 12.0:75.0 |
| C6H5 | CH(OH)C6H5 | 0.05 |
9 h | 10.8:67.5 |
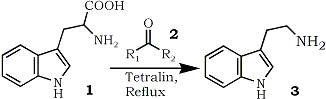
Of various methods available1, the decarboxylation of tryptophan (1) is the simplest way to the synthesis of tryptamine (3) which is a very important starting material in the synthesis of various indole alkaloids. Although there have been reported some syntheses of tryptamine (3) by the decarboxylation of tryptophan (1)2, besides unavoidable tedious isolating operations, use of a large excess of high boiling solvents such as diphenyl ether or diphenylmethane made these methods preparatively unfavorable. In 1964, Chatelus3 observed smooth evolution of carbon dioxide when certain α-amino acids including tryptophan (1) were heated with an excess of ketonic compounds forming the corresponding amines. However, this could not be used for the preparative purpose, since the amines formed were readily condensed with ketones existed in the reaction medium. It was now found that a modification of this observation provided a novel preparative method for the synthesis of tryptamine (3) by the decarboxylation of tryptophan (1).
The procedure involved the heating of tryptophan (1) in a relatively small amount of tetralin with catalytic amount of carbonyl compounds (2) as shown in the table until the evolution of carbon dioxide was ceased. Tryptamine (3) thus formed was cleanly distilled from the reaction mixture and a single crystallization yielded a pure product in good yield.
Experimental
General procedure
A suspension of L- or DL-tryptophan4 (1) (20.4 g, 0.1 mole5) in tetralin (200 ml)5 was refluxed in the presence of a carbonyl compound (2) with vigorous stirring. After the evolution of carbon dioxide was ceased and the reaction mixture became clear, the solvent was removed under vacuum by using rotary evaporator. The residue was distilled under reduced pressure to give a yellow crystalline solid, bp 140-155°C/0.25 torr. (lit.6 137°C/0.15 torr.), which was crystallized from boiling benzene to afford faint yellow prisms, mp 116-117.5°C (lit.7 115-117°C).
Preparative procedure
L- or DL- Tryptophan (1) 102.1 g (0.5 mol) was suspended in tetralin (250 ml) containing pentan-3-one 4.3 g (0.05 mol) and the mixture was heated to reflux for 8-10 hr with vigorous stirring until no more carbon dioxide was evolved. The reaction mixture was treated as above to give pure tryptamine (3) 67.1g (83.9 %)8.