(Quoted from page 3476:) Interestingly, very little has been reported on the Wacker oxidation of disubstituted olefins13. Its use in organic synthesis has been mainly limited to conversion of monosubstituted olefins into methyl ketones14. The regioselectivity observed in our case, namely exclusive oxidation of the benzylic rather than the homobenzylic carbon, is fortunately the desired one. Nevertheless, this situation is quite surprising on grounds of the known stereochemical course15 and expected regioselectivity of the Wacker process. It has been demonstrated both theoretically16a and experimentally16b that a nucleophile approaching a coordinated olefin from the face oppite to the metal is expected to attack the carbon that is substituted by an electron-donating group (or the position remote from an electron-withdrawing substituent). This tendency has been demonstrated in the Wacker oxidation of indene to β-indanone17a and of para-substituted styrenes to acetophenone and phenylacetaldehydes17b.
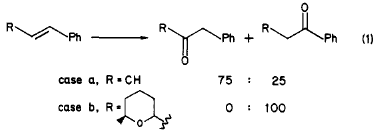
Indeed, we also found this prediction to be valid in the case of a Wacker oxidation of a model substrate, β-methylstyrene, which yielded phenylacetone and propiophenone in a 3:1 ratio (case a in eq 1). However, when this oxidation was repeated with β-tetrahydropyranyl styrenes (case b in eq 1), we observed the opposite regioselectivity, leading to the phenyl ketones exclusively.
Experimental
Wacker Oxidation of β-Methylstyrene
β-Methylstyrene (107 mg, 0.9 mmol), cuprous chloride (82 mg), and PdCl2 (15 mg, 0.08 mmol)
were mixed with DMF