11-20-01 14:11
No 238753
A long, long time ago, Mormon_Trailer sent me a few pictures (generated by the synthesis planning utility SynGen), which are synthetic outlines for a method of producing MDMA starting with only aliphatic precursors, forming the benzene ring as the last step. I have no idea about the synthetic details of each step, nor does the program, It just knows that the starting materials are commercially available (and calculates the cost of producing one mole of the target compound), and the general reported yield of similar transformations reported in the chemical literature.
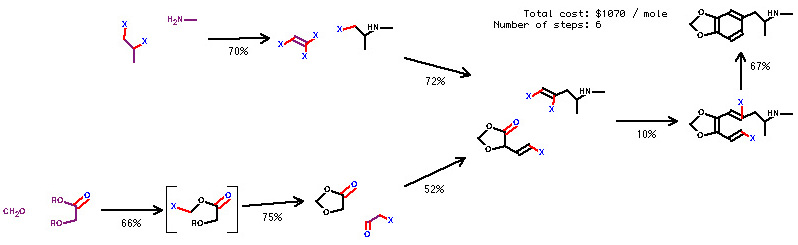
Again, this is just a digital brainstorm from a computer on how the synthesis could be done, and the synthetic details has to be worked out by any aspiring chemist by comparing each step with similar reactions in the literature. The above picture is just one (the cheapest) of nine different routes, the rest can be found at ../rhodium/archive/syngen/in
Any comments? Has anyone here ever built a benzene ring from scratch?
(Hive Bee)
11-24-01 11:26
No 239955
I have had this in mind for a long time and brought it up somewhere at the hive recently, but it didn't get much reply, probably because I hadn't done a nice piece of homework like Rhodium did here with his example and so posted it on a "less-than-serious" board.
This opens up many corridors of thought for two reasons: 1-good possibility of legally dodging the scheduled chemicals and their designer variants and 2-it allows a significantly wide variety of starting materials and reactive pathways, the latter enabling a larger population of people(read:skill levels) to participate in designing syntheses since we're no longer locked into the relatively small group of methods remaining , save for the new and exotic ones of course.
This participationability at the very least will help educate newbees by encouraging thought because we could use a lot of the standard reactions and reagents we learned in organic class, it would be like those "design a synthesis for x (no pun intended) problems" in our texts and on exams.
As for the analogue laws(at least in USA), it is the defining carbon skeleton of the end product and precursors thatare banned regardless of moieties attached. If one looks at the building of our fave molecules, they always begin with aquiring and manipulating the benzyl ring, substituted or not.It would seem to reason then(not that I'd bet 20 years on it though) that with the ring open the compound wouln't fit the definition of an "analogue or isomer" of a controlled substance. Looking at the Journal of Forensic Science articles(that continually state how unskilled and uncreative clandestine chemists are) I couldn't find any examples of lab busts or analyses of seized product that indicate non-cyclic pathways to said contraband. Coupled with the non-listed status of "open" versions of banned moleecules it would seem that, in the absence of finished product or strong circumstantial evidence(i.e. lab notebooks) one could very possibly beat the proverbial rap. At least the first person might, because in the wake they would surely try to list everything else they possibly could. Given how dumb they think clandestine cooks are, it is doubtful they would list every compound we discuss being possible to perhaps remotely be applicable to theoretical syntheses. I'll avoid making a roadkill reference in this forum.
Leaving ring closure to as late a step as possible adds to the margin of legal safety.
Just some food for thought: Alkynes open a door to lots of useful carbon-skeleton building, since acetylene is pretty darn un-stoppable OTC. Aside from electrophilic additions(though somwhat less reactive in this way than equivalent alkenes for reasons I do not know), alkynes undergo nice nucleophilic additions that simple alkenes do not like to. Terminal alkynes have acidic hydrogens that lend themselves to forming metal acetylides, sodium acetylides being not only good for making higher alkynes, but the acetylide ion is a stronger base than the hydroxide ion.
Rhod: This computer progran that helps brainstorm synths, where can I find more stuff like this? It seems that it would save a good deal of book-hounding, or at least suggest some options that one might not immediately think of. The cost-analysis is an excellent feature for identifying possible hurdles.
Ring building seems to allow many options not available to those who have one in the pot from the start. Seems to allow great flexibility as to which functional groups to work with and therefore provide options regarding one's reagent availability. There's always enol chemistry to fool with and the myriad of condensation reactions. Also, the order in which the molecule is built should allow more options for protecting sensitive groups in case one needs to get a little vigorous.
I haven't been able to find much in texts on rolling your owm aryl ring, it seems chemists always start with one in some form or another and add to it. This will be exciting to research in the literature. I'll keep y'all posted if I find anything that looks useful.
May Kekule's snake bite it's own tail at the end of your day...
(Hive Bee)
11-24-01 14:30
No 239986
Rhodium we owe you a billion dollars . . .
I think it is true that having an MDMA-like substance with its ring open is not likely to be illegal (at least for now). These routes are definitely worth looking into. I am not sure I understand the very last step in most of the processes shown. I've never seen references to ring-closing reactions that would accomplish the final step in each of those routes. I am going to try to do some research right now. Does anyone even know what that second-to-the-last substance might be called?
(Hive Bee)
11-25-01 08:18
No 240254
Hey Rhodium, skip extasy, try the five cheapest method off that program for LSD. after reading the commonly available methods of LSD production, those 5 are so, so tempting. but alas, aurelius knows too little chemistry to accomplish the task at this stage in the game.
(Chief Bee)
11-25-01 11:58
No 240291
Here is a $4000/mole three-step lysergic acid route, and another for $3000/mole: http://syngen2.chem.brandeis.edu/syngen.
(Hive Bee)
11-26-01 13:20
No 240736
Without accounting for stereochmistry, which is partially ambigous in this drawing, I would dare call it(numbered clockwise): calling "X" bromine, because I dislike being ambigous (2-halo, nah!):
1-bromo-3,4-methylenedioxy,6-bromo, 8-methylamino, non-1,3,5-ene
As for stereochemistry, the 1,2 double bond is drawn trans, but the one at5,6 is not drawn in a conventional way, the bond angles could't bee like that. Perhaps it's a limitation of 2-D drawing, but none the less it's ambigous. If I had to guess, I would draw the halogen straight down, rather than up, but there could be many arguements deepending on the previous chemistry.
Without the hydrogen at position 8, One cannot call it an R- or S- stereocenter, though I guess If I get off my lazy ass and look up Rhodium's references, I could figure it all out from the reactions, but, hey, I just wanted a crack at practicing my nomenclature.
yours truly,
1,3,-Boz-8-yne
(Chief Bee)
01-22-02 15:00
No 259366
Check out Harrison, Ernest A., Jr., "Benzodioxole Chemistry: Preparation and Selected Reactions of 3a,4,7,7a-Tetrahydro-4,7-methano-1,3-ben
(Master Searcher)
01-23-02 01:40
No 259603
>and the references sited therein.
BTW, you might have noticed that US patents use prepositional compounds like "therein", "herein", "thereon", etc. a lot whereas they tend not to be used anywhere else to that extent. After seeing this I found that it gave me some insight into the more frequent use of prepositional compounds in German, ie. daron, davon, darin, daraus, damit, herin, herunter, wohin, etc. It might help to know when translating German to English.
http://www.geocities.com/dritte123/PSPF.
(Newbee)
02-10-02 22:11
No 267793
What caught my eye was the second step in making the methylenedioxy bridge. Using the same procedure on eugenol would give our beloved safrole, correct? Why cleave the methoxy group at all when you can whip it into the end product? If you can't beat 'em, join 'em, right?
I sell crack for the CIA
(Newbee)
02-16-02 22:26
No 270555
Come one bees! We need some input! That step says that the ring starts with Oxygens in the 3,4 positions, with, according to the picture, ANY group attached to the 4 oxy, and a carbon and an 'X' attached to the 3 oxy. Now I'm assuming that that 'X' is a halogen, and if it is, could not eugenol be substituted in the catechol-->safrole synth, ../rhodium /safrole
Or does anyone have an article outlining that particular reaction in the pic, as it gives a yeild of 75%!! That's fuckin' amazing given eugenol's availability.
I sell crack for the CIA
(Newbee)
02-27-02 11:34
No 274179
We wanna have some extended demo versions of this program 'syngen'!
Disclaimer: everything I said I pulled outta my arse
(Hive Bee)
03-11-02 07:17
No 280479
Well, this post isn't going anywhere, but I have been thinking about this. I believe that when choosing the halide for the methoxy-hydroxy combination trick, iodine should be avoided, because of it's tendency to form radicals that undergo substitutions with an oxygen when the oxy is attached to a carbon, examples, HI reflux has been proven to kill methoxy groups, methylene dioxy chains, the good old HI/RP reaction, well, that's all I can think of. But my point is, if any bees are payin, attention, I think experiments should be limited to Br or even Cl.
I have thought about this, though. Could a high yeilding, easy eugenol->safrole synth be as easy as refluxing in Br and PTC? Comments?
I sell crack for the CIA