(Chief Bee)
06-04-03 01:10
No 437649
(Rated as: excellent)
I feel that this crystallographic study may shed some light on as to why the potency of the MDMA stereoisomers is the reverse of MDA and analogs...
Ecstasy: 3,4-Methylenedioxymethamphetamine (MDMA)
Acta Crystallographica C54, 229-231 (1998) (../rhodium/pdf /mdma.crystal
There are several structure-activity relationships which clearly differentiate MDMA from other hallucinogenic amphetamines, such as DOB (2,5-dimethoxy-4-bromoamphetamine) or DOM (2,5-dimethoxy-4- methylamphetamine). N-Methylation of hallucinogenic amphetamines attenuates activity three- to tenfold and the R(-) configuration is more potent. For MDMA, N-methylation has very little effect on activity and the S(+) configuration is more potent. The structure-activity relationship of MDMA is different even when compared with MDA (3,4-methylenedioxyamphetamine), which lacks N-methylation. For MDA, the R(-) isomer has the greatest potency in an in vivo rabbit model for classical hallucinogenic activity, whereas the S(+) isomer, at the same dose as the R(-) isomer, has a greater effect on emotion and empathy.
The structure of MDMA is illustrated in Fig. 1. The methylenedioxy ring is essentially coplanar [0.7°] with the phenyl ring. One interesting structural feature of MDMA is the orientation of the isopropylamine group. In MDMA, the torsion angle which describes the relationship of the
 -methyl group (C10) and the phenyl ring is -66.4°. This is unlike other hallucinogenic amphetamines, such as DOET (2,5-dimethoxy-4-ethyl-amphetamine), where the
-methyl group (C10) and the phenyl ring is -66.4°. This is unlike other hallucinogenic amphetamines, such as DOET (2,5-dimethoxy-4-ethyl-amphetamine), where the  -methyl group is antiplanar with a torsion angle of 178°, and TMA (2,4,5-trimethoxyamphetamine), where the angle formed by the
-methyl group is antiplanar with a torsion angle of 178°, and TMA (2,4,5-trimethoxyamphetamine), where the angle formed by the  -methyl group is 170°.
-methyl group is 170°.The relative position of the amino N atom is also different for MDMA when compared with DOET or TMA. For MDMA, N-methylation results in a rotation about the C8---C9 bond, giving rise to a torsion angle between the
 -methyl (C10) and N-methyl (C11) groups of 170.0°. When comparing the structure of MDMA with DOET or TMA, it appears that the relative position of the
-methyl (C10) and N-methyl (C11) groups of 170.0°. When comparing the structure of MDMA with DOET or TMA, it appears that the relative position of the  -methyl group (C10) and the amino N atom (N1) are transposed.
-methyl group (C10) and the amino N atom (N1) are transposed.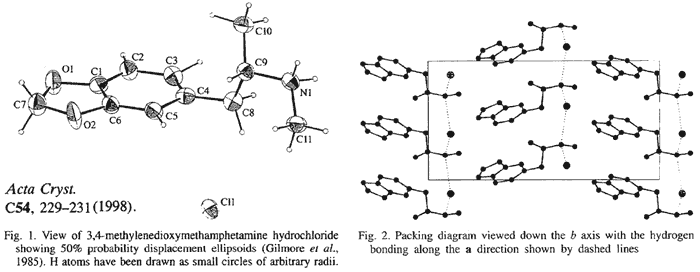
(Moderator)
06-04-03 11:50
No 437747
The crystal structure of the hydrochlorides of such simple molecules has nothing to do with the receptor bound conformation.
The difference between R and S stereoisomers has most probably to do with the different molecular targets (5-HT receptor vs. 5-HT transporter).
(Chief Bee)
06-05-03 00:31
No 437830
Yes, but why do MDA and MDMA have different molecular targets despite the small difference in structure?
I checked the conformation of MDA vs. MDMA after doing an energy minimization of the structures in ChemOffice 3D, and it gave approximately the same results as the crystallographic study above - the molecule wants to be in its lowest energy conformation both in solution and in a crystal (even though it may differ between the two) - therefore (S)-MDA and (R)-MDMA are more alike in the solution phase, and the 5-HT transporter wants to have the side chain bent in that fashion, therefore these two isomers are the most 5-HT releasing ones...
(Hive Addict)
06-05-03 21:52
No 438055
This should be an "excellent" thread. (I want to be able to find it under the positive rating threads. Not to mention it simply deserves that rating.)