10-10-03 08:57
No 463737
In Tihkal, Shulgin states:
A suspension of 3.15 g d-lysergic acid hydrate and 7.1 g of diethylamine in 150 mL CHCl3 was brought to reflux with stirring. With the external heating removed, there was added 3.4 g POCl3 over the course of 2 min...
If I have done the math correctly, 3.15 g of d-lysergic acid hydrate is 3.15/300.332*1000 = 10.5 mmol, 7.1 g of diethylamine is 7.1/72.129*1000 = 98.4 mmol, and 3.4 g of phosphorus oxychloride is 3.4/153.332*1000 = 22.2 mmol. I believe that there should be 2 moles of POCl3 for every mole of d-lysergic acid hydrate--one mole to react with the hydrate part and one mole to chlorinate the lysergic acid. Is there a reason that he used a slight excess? Why did he use so much diethylamine?
Oxygen69
It really isn't that hard, you know?
(Chief Bee)
10-10-03 10:10
No 463745
Is there a reason that he used a slight excess?
Probably to compensate for any side reactions, water content the other reagents used, atmospheric moisture etc.
Why did he use so much diethylamine?
Because it's dirt cheap compared to lysergic acid, and he wanted to make sure that all all the latter molecules would get a chance to react. Some of the diethylamine will also be tied up as relatively unreactive salts with H2PO2Cl2 and other oxyacids formed by the reaction of POCl3 with water and lysergic acid. Some might even form phosphorous diethylamides with the POCl3 directly, but I'm not sure.
(Hive Addict)
10-24-03 00:22
No 466405
Route is tha Lysergic acid is easily fouled by attempts to make the acid chloride and Sn2 with diethylamine exept in the case of POCL3
makes one wonder why this method works best?
you know when my old man was in college in organic back in the 60's this was the method he was shown by his prof before the feds confiscated all the lysergic acid from the universities, it seems to be methos # 1 among all the chemists.
I like the SO3 method too because you can use the monohydrate.
the advatantage of the POCl3 method is the one-pot shot one step reaction convenience of it.
(Chief Bee)
10-24-03 17:25
No 466517
Check out the first page of the article linked in Post 465132 (Rhodium: "Garbrecht's Classic LSD Synthesis", Tryptamine Chemistry)
The classical methods for preparing amides by acylation of amines with esters or acid chlorides fail when applied to lysergic acid. Thus, while the methyl and ethyl esters of lysergic acid are known, they fail to undergo aminolysis except in the special case already mentioned involving the use of hydrazine. On the other hand, attempts to prepare lysergic acid chloride yield only decomposition products.
(Hive Addict)
10-30-03 08:22
No 467766
to using the hydrazide route is that you can isolate rather pure lysergic acid hydrazide as rhombic needles and wash the impurities away with solvents this would be the method of choice when working with natururally occuring lysergamides, e.g. the ipomea family (morning glories) contain clavine alkaloids that are nearly impossible to seperate other than via the hydrazide.
another way from the hydrazide is the formation of the dimethylpyrazole but you probably know this stuff.
one possible reason POCL3 works is that the acid chloride is formed in situ and immediately displaced by diethylamine which could explain the lesser decomposition using this method.
but that azide method looks cool.
(Stranger)
11-01-03 11:08
No 468091
The hydrazide method looks interesting and has its advantages, but anhydrous hydrazine is awfully nasty stuff--low-boiling, carcinogenic, poisonous, and explosive.
..and it tastes like licorice!
(Hive Bee)
01-28-04 01:49
No 485002
Why did he use so much diethylamine?
The excess diethylamine is used to neutralize the HCl that would be formed otherwise. 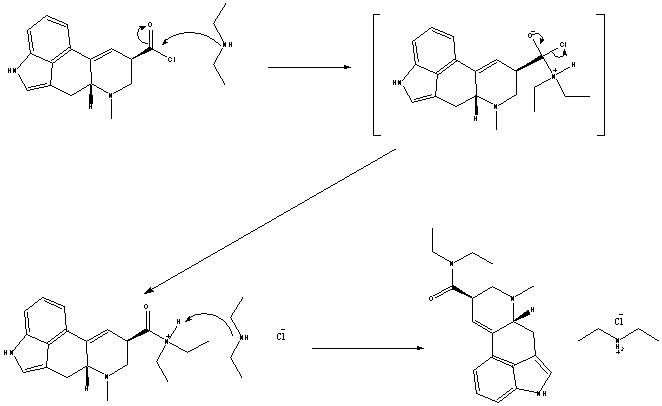
Hippler