(Hive Bee)
05-01-04 10:19
No 504206
(Rated as: excellent)
A Direct Access to 3-(2-Oxoalkyl)indoles via Aluminum Chloride Induced C-C Bond Formation
Manojit Pal, Rambabu Dakarapu, and Srinivas Padakanti
J. Org. Chem., 2004, 69(8), 2913-2916
DOI:10.1021/jo049923t

Supporting information (http://pubs.acs.org/subscribe/journals/
Abstract
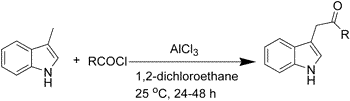
3-Methylindole is acylated regioselectively at the methyl group when treated with a variety of acyl chlorides in 1,2-dichloroethane in the presence of AlCl3, affording a mild and direct method for the synthesis of 3-(2-oxoalkyl)indoles. The product formation in this one-pot reaction largely depends on the conditions of the reaction employed. The methodology does not require protection-deprotection steps and is amenable for the scale-up synthesis of these indole derivatives.
[...]
Typical procedure for the preparation of 1-(1H-3-Indolyl)-2-propanone (3a):
To a solution of 3-methylindole 1 (3g, 22.9 mmol) in dry 1,2-dichloroethane (40 mL) was added fused AlCl3 (9.1g, 68.6 mmol) at 0 şC. The mixture was warmed to 25 şC with vigorous stirring and the stirring continued for 30 minutes at the same temperature. After cooling the mixture to 0 şC acetyl chloride 2a (2.0g, 25.6 mmol) was added slowly and drop-wise. The mixture was stirred at 25 şC for 48 h. and then poured into crushed ice (80g) slowly and portion wise. The mixture was stirred until it reaches to room temperature and extracted with methylene chloride (4 x 25 mL). The organic layers were collected, combined, washed with brine (2 x 25 mL), dried over anhydrous Na2SO4 and concentrated. The residue thus obtained was purified by column chromatography using petroleum ether-ethyl acetate to give the title compound (2.3g, yield 58%).
3-Methylindole stinks like shit (literally) and indole-3-yl-acetone is an excellent precursor for AMT.
The tendency is to push it as far as you can