09-19-04 20:42
No 532189
In the original "N,N-Carbonyl diimidazole" process for making LSD, (see Post 395675 (Rhodium: "English translation (Yield of LSD freebase 57.5%)", Tryptamine Chemistry) ) almost equi-molar amounts of Lysergic Acid and Diethylamine were used:
"(5mmol) anhydrous D-Lysergic Acid... was mixed with… a solution of 0.40g (5.5mmol) Diethylamine in 5ml DMF”.
Yet, the same procedure listed in various new Patents (see Patent US6476199 and Patent US6063908 )specify a 10x molar ratio of Diethylamine to Lysergic Acid, quite a large difference!
Is there any good solid reason to use such a large ratio of Diethylamine?
Other procedures, such as the "POCl3-Shulgin" method only use 5x amounts of Diethylamine.
Any comments? Thanks.
(Hive Bee)
09-21-04 13:22
No 532481
Could someone lay out what occurs in the N,N-Carbonyl diimidazole procedure? I'm a cook, not a chemist!
(Hive Bee)
09-21-04 16:35
No 532499
Not sure if the CDI method is like the peptide coupling methods, but in peptide coupling methods only about 2 times excess amine to be reacted is utilized. The CDI overkill excess amine could possibly be to make sure that all of it reacts, as it's better to have leftover worthless amine rather than unreacted product, eh? (all methods have some ratio of over-excess amine, for this purpose, SWIM thinks.)
(Chief Bee)
09-22-04 05:30
No 532624
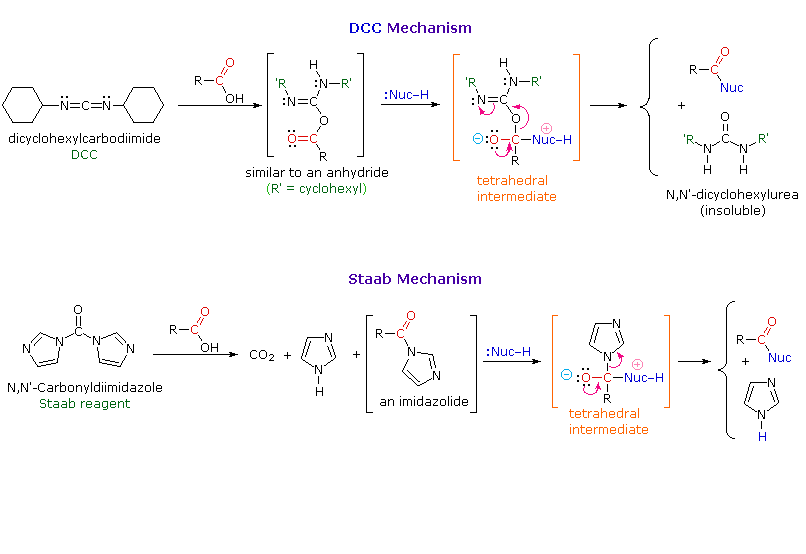
Source: http://www.cem.msu.edu/~reusch/VirtualTe
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
09-23-04 03:36
No 532769
Good info and References:
http://www.albmolecular.com/features/tek
(Hive Bee)
10-09-04 14:28
No 535084
(Rated as: good read)
A few articles I've run across regarding Peptide Coupling Agents, (for instance http://www.peptide-and-dna.com/marder.pd
it is suggested that Choloroform or DCM may be better solvents to carry out the reaction in. The above article says "If the activation is carried out in a solvent of low dielectric constant such as CHCl3 or CH2Cl2, the formation of {the carbodiimide} occurs instaneously...... However, if the activation is carried out in a more Polar solvent such as DMF, no immediate reaction can be detected and a complex mixture..."
My question is, while the article is about Peptide coupling, would this also be applicable to the Lysergic Acid/N,N-Carbonyldiimidazole reaction?