04-15-02 09:47
No 297224
Ethylene glycol + HCl > Ethylene Chlorohydrin
Ethylene + Cl2 and H20 > Ethylene Chlorohydrin
Ethylene Chlorohydrin + Alkali > Ethylene Oxide
Catalytic Isomerization to Acetaldehyde
Aluminum oxide (Al2O3), phosphoric acid and phosphates, iron oxides, and, under certain conditions, silver, catalyze the isomerization of EO to acetaldehyde.
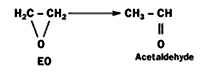
More Info
Post 297178 (Elementary: "Alkylene Oxides from Glycols", Novel Discourse)
http://www.ethyleneoxide.com/html/body_p
Patent GB1036130
Nobodys home
(Old P2P Cook)
04-15-02 09:53
No 297231
Seems a tough route to take to make acetaldehyde. Most people would probably just oxidize ethyl alcohol.
(Hive Addict)
04-15-02 10:00
No 297237
Ethylene glycol is a lot cheaper than ethanol (no duty)
Nobodys home
(Hive Prodigy)
04-15-02 13:38
No 297338
You dont have stabdard 70% EtOH as a household cleaner/antiseptic?
PrimoPyro
Vivent Longtemps La Ruche!
(Hive Addict)
04-15-02 14:21
No 297362
We have vodka and methylated varieties only, not even everclear available to the common household.
(as far as I know !)
Nobodys home
(Hive Prodigy)
04-15-02 14:38
No 297366
Ok. As my last offtopic suggestion for the casual reader, I then think that it would be much easier to brew ethanol from fermentation of sugars and baker's yiest, followed by distillation, and weak oxidation to acetaldehyde.
Just my opinion on feasability. And with that, I must say that your idea is pretty clever.
PrimoPyro
Vivent Longtemps La Ruche!
(Master Searcher)
04-15-02 16:28
No 297400
Could you do a pinocol rearrangement of ethylene glycol to get acetaldehyde?
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Stranger)
04-17-02 01:25
No 298089
From the ethylene oxide MSDS:
explosive limits: 3-100%, flash point <-18°C, boiling point 10.4°C
They use ethylene oxide as the fuel in these big fuel-air-bombs.
It's also said to be incompatible to temps above 52°C, oxidizers, ALKALIS, acids, alkohols, mercaptams, copper, SILVER, magnesium, mercury and their salts.