(Hive Addict)
06-18-04 18:28
No 514141
(Rated as: excellent)
J.Med.Chem. - 24,8.1981;940
4-allyloxy-3,5-dimethoxybenzaldehyde
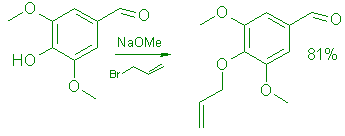
To 18.2g (0.1 mol) of syringic aldehyde in 40 mL of MeOH was added a solution of 6g (0.11 mol) of NaOMe in 35mL of MeOH. A clear yellow solution was formed. Allyl bromide (15.4g 0.13mol) was added, then the mixture was refluxed for 1.5h. A clear solution was formed within 15 min. The solvent was removed in vacuo and the residue recrystallized twice from hexane: yield 18g (81%)M mp 45-46°C
Alkylation with allyl tosylate should also be considered. TsO-Et=Me should be easy to prepare by the procedure outlined in Post 492515 (Vitus_Verdegast: "Co(II) catalysed tosylation of alcohols", Chemistry Discourse).
PiHKAL #2 AL is a definite winner with the 3C analog yet to be tested.
 J.Chem.Soc., Perkin Trans.2, 2000, 1119-1123
J.Chem.Soc., Perkin Trans.2, 2000, 1119-11235-bromoveratraldehyde by solid phase bromination of veratraldehyde with NBS damn intresting article, lots of other aldehydes brominated.

Synthetic Communications 20;17;1990;2659-2666 - the original article can be found in Post 514124 (not existing)
5-Bromovanillin (5).
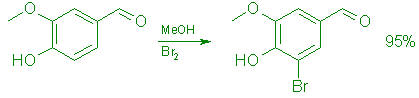
To a stirred, cooled (0°C) solution of 152.15 g (1.0 mol) of vanillin in 1.0 L of methanol was added during 20 min 176.0 g (1.1 mol) of bromine at such a rate that the temperature was kept below 20°C. The mixture was stirred at room temperature for 1 h, cooled to 0°C, and treated during 30 min with 500 mL of cold (5°C) water. Stirring was continued for 15 min and the product was collected by filtration. It was washed with water (4 x 500 mL), then with 500 mL of cold (0°C), 70% methanol, and dried in vacuo at 50°C overnight to give 218.5 g (95%) of 5 as pale yellow crystals, mp 163-164°C (Lit.,7 mp 163-164°C); UV (EtOH): 357 (E=4,200), 292 (e=9,800), 235 (e=15,300), 215 (e=19,000) nm; IR (CHCl3): 3300 (OH), 1675 (C=0) cm-1; 1H NMR (Me2SO-d6): S 3.93 (3 H, s), 7.35
(1 H, d, J=Hz), 7.60 (1 H, d, J=l Hz), 9.74 (1 H, s), and 10.51 (1 H, s, OH); MS m/z 230 (100, M+).
Anal. Calcd. for C8H7BrO3: C, 41.59; H, 3.05; Br, 34.59. Found: C, 41.48; H, 3.33; Br, 34.87.
Syringaldehyde (7) :
A: From 5-Bromovanillin (5).
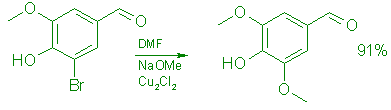
Under a blanket of argon, a solution of 214 g (0.93 mol) of 5-bromovanillin in 420 mL of anhydrous DMF was added during 25 min to a stirred slurry of freshly prepared, powdered sodium methoxide (3.72 mol, from 85.2 g of clean sodium in 1.0 L of CH3OH, followed by removal of the excess CH30H by distillation under vacuum) and 10.7 g (0.054 mol) of freshly prepared cuprous chloride in 150 mL of anhydrous DMF. The mixture was stirred at 97°C for 2.5 h, whereupon the color changed from blue to green after 30 min, and then to beige after 45 min. It was cooled to 60°C, and the DMF was evaporated under vacuum (0.2 Torr). To the residue was added 1.0 L of 15% brine and the mixture was stirred at 50°C for 30 min, cooled to 0°C and slowly acidified with 300 mL of cold (0°C) conc. hydrochloric acid. Stirring was continued at room temperature for 1.0 h and the crude product was collected by filtration. It was washed with 4 x 400 mL of cold H20 until neutral and extracted with warm (60°C) ethyl acetate (5 x 500 mL). The extract was evaporated to give 154 g (91%) of syringaldehyde, mp 109 111°C (t,it.,8 109-110°C); TLC (60:30:1 ethyl acetate:hexane:formic acid) showed only one spot, Rf 0.55; 5 had Rf 0.70. UV (EtOH): 365 (sh, e=1,200), 310 (e=13,100), 231 (e=15,980), 212 (E=18,500) nm; IR (CHCl3): 3520 (OH), 1685 (C=O); 1H NMR (CDC13 ): S 3.90 (6 H, s), 6.40 (1 H, s, OH), 7.10 (2 H, s), 9.75 (1 H, s); MS: m/z 182 (100, M+), 167 (12, M-CH3).
Anal. Calcd. for C9H1004: C, 59.34; H, 5.53. Found: C, 59.43; H, 5.28. Syringaldehyde prepared above may be crystallized from ethyl acetate (93% return) to give pale yellow crystals, mp 110-112°C.
B: From 3,5-Dibromo-4-hydroxybenzaldehyde (6).
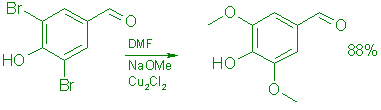
A 165-g (0.59 mol) batch of 6 in 156 mL of methanol and 312 mL of anhydrous DMF was reacted during 4.0 h with 4.78 moles of freshly prepared sodium methoxide in the presence of 9.45 g (0.047 mol) of cuprous chloride under the conditions described previously for the conversion of 5 to give 153.3 g (88% yield) of 7, mp 105-108°C.
3,4-Dibromo-4-hydroxybenzaldehyde:
A: From 4-Hydroxybenzaldehyde.
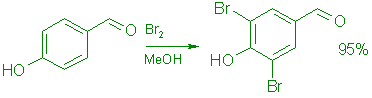
To a stirred, cooled (0°C) solution of 122.0 g (1.0 mol) 4-hydroxybenzaldehyde in 1.0 L of methanol was added during 30 min 325.0 g (2.2 mol) of bromine at such a rate that the temperature was kept below 20°C. The mixture was stirred at room temperature for 1.0 h, 800 mL of methanol was removed by distillation (water aspirator) at 50°C, and to the warm (45°C) solution was added 2.0 L of water during 20 min. The mixture was stirred at 0°C for 1.0 h and the product was collected by filtration. It was washed with water (5 x 1.0 L) and then with 500 mL of cold, 30% aqueous methanol, dried in vacuo at 70°C for 18 h to give 264.4 g (95.5%) of 6 as a colorless powder, mp 180-182°C. TLC (silica gel, 30:60:1 ethyl acetate: hexane: formic acid) showed only one spot under short wavelength UV light. UV (EtOH): 346 (E=8,000), 278 (E=9,000), and 221 (e=18,600) nm; IR (CHCl3): 3335, 1675, 1506, and 855 cm-1; 1H NMR (Me2SOd6) S 7.96 (2 H, s), 9.75 (1 H, s), and 10.23 (1 H, br, OH); MS: m/z 280 (M+). Anal. Calcd. for C7H4Br2O2: C, 30.04; H, 1.44; Br, 57.09. Found: C, 30.14; H, 1.49; Br, 56.94.
B: From p-Cresol.
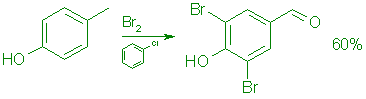
A cooled (10°C), stirred solution of 108.1 g (1.0 mol) of pcresol in 500 mL of chlorobenzene was treated during 30 min with 720 g (4.55 moles) of bromine in 600 mL of chlorobenzene at such a rate that the temperature was kept below 25°C. The mixture was then stirred at reflux for 4.5 h and concentrated in vacuo at 60°C to give 219 g of a red oil. This was dissolved in 500 mL of methanol, cooled to 5°C, and treated with 500 mL of 1.ON hydrochloric acid. The mixture was stirred at 5°C for 4.5 h, diluted with 1.0 L of cold water, and the product was collected by filtration. It was washed with 500 mL of cold 50% aqueous methanol and dried in vacuo at 35°C overnight to give 168.3 g (60%) of 6, mp 179-182°C.
UV (EtOH): 346 (e=8,000), 278 (e=9,000), and 220 (e=19,100) nm; 1H NMR (CDC13): S 6.43 (1 H, s, OH), 7.89 (2H, s), and 9.75 (1 H, s); MS: m/z 280 (100, M+).
Anal. Calcd for C7H4Br2O2: C, 30.04; H, 1.44; Br, 57.09. Found: C, 30.27; H, 1.65; Br, 56.94.
3,4,5-Trimethoxybenzaldehyde (1).
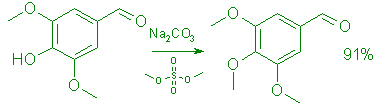
A stirred mixture of 153.0 g (0.84 mol) of syringaldehyde (7) in 1.0 L of acetone, was treated with 127.0 g (1.02 mol) of dimethyl sulfate, 118.0 g (1.11 mol) of anhyd. Na2CO3, and 3.0 g (0.053 mol) of KOH in 32 mL of water. The mixture was stirred under reflux for 18 h. cooled to room temperature and filtered. The filter cake was washed with acetone (3 x 200 mL) and the filtrate and washings were concentrated to ca. 200 mL. The stirred solution was diluted with 500 mL of water during 30 min, followed by 1.5 L of water during 15 min. Stirring was continued at room temperature for 15 min and then at 0°C for 30 min, and the product was collected by filtration. It was washed with water (4 x 2.0 L) and dried in vacuo at 25°C for 24 h to give 150.2 (91.5%) of 1 as colorless crystals, mp 73-75°C (Lit.,9 74°C). TLC (4:1 ether-hexane) revealed only one spot, Rf 0.57. UV (EtOH): 218 (e=25,400) and 287 (E=10,450) nm; IR (CHCl3): 1695 cm-1; 1H NMR (CDC13): S 3.89 (9 H, s), 7.09 (2 H, s), 9.81 (1 H, s); 13C (CDC13) S 56.07 (2 C, q, 2 x OCH3), 60.74 (1 H, q, OCH3), 106.55 (2 C, d, 2 x C=CH), 132.1 (1 C, s, ArC), 143.8 (1 C, s, ArC), 154.0 (2 C, s, 2 x ArC), 190.82 (1 C, d, CHO); MS: m/z 196 (M+).
Anal. Calcd. for C1OH1204: C, 61.22; H, 6.17. Found: C, 61.27; H, 6.24.
--psyloxy--
(Hive Addict)
06-21-04 23:39
No 514665
Since 4-methoxy-3,5-dibromophenethylamine is reported (by G_Pig) to be an active hallucinogen, I think 5-bromo-3,4-dimethoxy-PEA/A should be looked into.
Chem.Pharm.Bull.;11.1963;1500
ß-nitro-5-bromo-3,4-dimethoxystyrene
A mixture of 60g of 5-bromoveratraldehyde, 50ml nitromethane, 20g NH4AcO and 160ml AcOH was refluxed for 2h. The reaction mixture was then poured into 2L of ice water. The crystalline precipitate was collected and recrystallized from AcOEt yielding 39g (55%) of yellow needles, mp 157-158°C
--psyloxy--
(Hive Addict)
06-22-04 08:58
No 514735
The following reference might be of interest as well: Post 423331 (GC_MS: "additional information", Methods Discourse)
Aztecunnilingus Maximus
(Hive Addict)
06-22-04 18:16
No 514784
Since 4-methoxy-3,5-dibromophenethylamine is reported (by G_Pig) to be an active hallucinogen, I think 5-bromo-3,4-dimethoxy-PEA/A should be looked into.
Give me a month or so.. (I sure wish there were 48 hours in a day
http://www.psychedelicrepublicans.com/
(Hive Addict)
06-24-04 21:10
No 515177
(Rated as: good read)
 Indian J.Chem.Sect.B, 24.1985;294-295
Indian J.Chem.Sect.B, 24.1985;294-2955-bromo-veratraldehyde to 3,4,5-trimethoxybenzaldehyde w/NaOMe, CuCl2 unfortunately they use an autoclave, so I don't think this example is of much use.
5-bromovanillin to 5-bromoveratraldehyde with DMS
Dimethyl sulphate (80mL) was added slowly to a stirred solution of 5-bromovanillin (21g) in 2.5% NaOH solution (150mL) while keeping the temperature between 30 and 35°C. After the addition was over, the solution was boiled for 2h, cooled in an ice-bath, and the precipitated product filtered and crystallised from 80% ethanol to get the title compound as flocculant crystals, yield 1gmust be a typo!.
--psyloxy--
(Hive Addict)
07-01-04 23:39
No 516790
Not the most complete description of a synthesis ever, but it seems to be the only example of an MeN2 methylation of syringaldehyde in the literature.
Chem.Pharm.Bull. - 32,2.1984;578-584
An ether solution of syringaldehyde was treated with diazomethane etherate and the mixture was allowed to stand at room temperature overnight. The solvent was evaporated to give 3,4,5-trimethoxybenzaldehyde.
--psyloxy--
(Hive Addict)
07-19-04 10:16
No 520232
J.Chem.Soc., Perkin Trans.2, 2000, 1119-1123
5-bromoveratraldehyde by solid phase bromination of veratraldehyde with NBS damn intresting article, lots of other aldehydes brominated.
Only now I noticed it... I thought the bromination of veratraldehyde yielded 2-bromo-4.5-dimethoxybenzaldehyde, or am I wrong?
../rhodium /tma2.va
Aztecunnilingus Maximus
(Hive Bee)
10-07-04 04:46
No 534774
Psyloxy, how did SWIP synth allyl bromide for the allylation? Via the phosphorus trihalide route? If not, then please explain in detail...SWID can not think of a method (certainly not using HBr and H2SO4) to synth this from allyl alcohol without reacting on the double bond. Thanks.
Drug Chemists are Ta to a good Sm.
(Chief Bee)
10-07-04 07:28
No 534790
KBr/H2SO4 should work, see ../rhodium /tcboe/c
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
10-07-04 23:04
No 534863
Oh, thanks Rhod, SWID was under the impression that the HBr formed in situ could easily add across the double bond. He will definetly give this a shot. (He also noted that the procedure outlined in the above link appears to be designed to keep the acid content low.)
On another note, somewhat modified by the preceding enlightening information, SWID is wondering if the nitrostyrene, with the allyl ether substituent, could survive a reduction via GAA/Al-Hg/IPA (../rhodium /nitrost
Drug Chemists are Ta to a good Sm.
(Chief Bee)
10-08-04 03:15
No 534881
Mechanism of the bromination of allyl alcohol with hydrobromic acid:
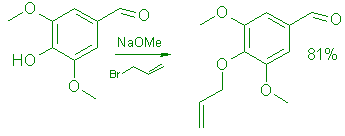
The allyl carbocation forms very easily in acid solution because is stabilized due to allylic charge delocalization (http://members.aol.com/profchm/conj.htm
This makes substitution of allylic structures undergo almost exclusively SN1 (http://www.wordiq.com/definition/Nucleo
As for the preparation of an allyloxy-substituted phenethylamine, you better react a phenolic benzaldehyde with allyl bromide and then turn that into the corresponding nitrostyrene and reduce to the amine like in ../rhodium /leminge
The Hive - Clandestine Chemists Without Borders
(Über-Führer die Ironie)
10-08-04 11:20
No 534938
Genrally speaking, when you alkylate with alkylbromides - do add catalytic amount of NaI or KI to the flask...
(Chief Bee)
10-19-04 02:30
No 536494
This article was originally posted in djvu format in Post 515177 (psyloxy: "Indian J.Chem.Sect.B, 24.1985;294-295", Chemistry Discourse)
Regiospecific Alkoxylation of Phenolic Aldehydes
3,4,5-Trimethoxybenzaldehyde from 5-Bromoveratraldehyde & Syringaldehyde from 5-Bromovanillin
S.C. Puri, S.M. Anand & C.K. Atal
Ind. J. Chem. 24B, 294-295 (1985) (../rhodium /vanill
Abstract
Regiospecific alkoxylation of bromophenolic aldehydes has been carried out. Vanillin (I) is brominated (Br2/AcOH) to 5-bromovanillin (II) which is subsequently methylated to give 5-bromoveratraldehyde (III). The alkoxylation of II and III with freshly prepared sodium methoxide in the presence of anhydrous cupric chloride and dimethylformamide furnishes 4-hydroxy-3,5-dimethoxybenzaldehyde (IV) and 3,4,5-trimethoxybenzaldehyde (V) respectively. Methylation of IV with dimethyl sulphate and anhyd. potassium carbonate in dry acetone gives V.
The Hive - Clandestine Chemists Without Borders