08-04-04 10:06
No 523590
Hi beez, I just noticed something, diquat weedkiller is usually as the dibromide salt, how about evaporating a bottle of diquat to powder, drying over NaOH or CaCl to prevent H2O forming HBr, and then treating with conc. H2SO4 or H3PO4? shouldn't this liberate elemental Br, which could then be condensed in a receiving vessel?
Kids, say NO to the DEA!
(Hive Bee)
08-04-04 12:01
No 523597
It's of relatively low toxicity to humans (unless mixed with paraquat) but I guess the concentration is quite low making it expensive as a source, what else is in it and in what concentrations? Possibly the liberated bromine will react with the organic components and/or their breakdown products thus reducing yield.
http://www.greenlightco.com/products/MSD
Fuck the creationists - MC Hawking
(Hive Bee)
08-04-04 12:22
No 523598
One major brand name preperation containing diquat is listed as 36.4% diquat dibromide, with the rest being listed simply as inert ingredients,
Just so all is covered, diquat is chemically, 1,1-ethylene2,2-dipyridylium dibromide.
Looks like there might well bee enough goodies in there to concentrate and try to extract useable quantities of bromine then.
Kids, say NO to the DEA!
(Hive Bee)
08-04-04 14:03
No 523608
'inert' ingredients to what though? it would mean inert in the sense it has no effect on the weedkiller aspect of the product.
What karl was pointing out is that bromine elemental (Br2) is pretty reactive so it would, once seperated, react with some other part of the molecule (maybe) or something else in the mixture and there goes your yield.
You could possibly try and convert to freebase and NaBr of the compound... as for bromine from NaBr, thats not to bad.
But i dont know how well this would work. you may end up with contaminated product with weedkiller, mix that kind of contamination with something your going to eat/inject/snort ??? have fun...
-AC
Its just my opinion, but no-one listens to me anyway, and rightly so...
(Chief Bee)
08-04-04 14:04
No 523609
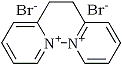
Diquat dibromide does not contain bromine, only two bromide ions.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
08-04-04 14:29
No 523612
Rhodium, I know there is no elemental Br in weedkiller, or in anything else I could think of that is on sale to the public.
I think the idea of trying to convert to NaBr would bee an interesting thing to try, after conversion to the group I bromide acidification with conc. H2SO4 should release the Br yes?
I came up with this because there is no pool supplier that I know of anywhere near my area, I don't know as the UK is known for them really, not warm enough for private pools, and I thought some bees might also bee able to benefit from this.
And besides it hadn't been covered in TFSE, so I suppose it's another possible source although I can't do any testing because I don't have access to my lab at the moment.
One other thing, it seems diquat is strongly attracted to clays and dirt because of it's charge, so much so, that it cannot bee used in muddy water, maybe this would bee a way to seperate out the diquat from solution, using diatomaceous clay or fullers earth etc.
Kids, say NO to the DEA!
(Hive Bee)
08-05-04 06:07
No 523756
i think you should find a better way to get it, this way would yield shit all (more than likely) for a high cost and a pain in the ass to manage the works.
So sorry, give up.... find another way.
-AC
Its just my opinion, but no-one listens to me anyway, and rightly so...
(Hive Bee)
08-05-04 23:19
No 523858
I wouldn't have even considered it myself, but I have absolutely ZERO access to ANY bromine containing compounds whatsoever, and worse, no pool suppliers near me that I know of at all.
Kids, say NO to the DEA!
(Hive Bee)
08-06-04 03:05
No 523903
If it is to be successful you must prise those bromine ions away from the organic components of that molecule before you oxidise it to be sure, if bromine is that scarce for you. Basifying with NaOH to generate an NaBr solution seems potentially like a good idea, unless the molecule does something weird at a high pH.
Fuck the creationists - MC Hawking
(Hive Bee)
08-06-04 03:10
No 523905
No point now, I now have a source for pool chems, although I might look into this just as an exercise and out of intererst.
My gratitude to the the kind person that revealed my source, naughty though it was LOL.
(Hive Bee)
08-06-04 04:05
No 523920
was about to say, not everything is close to hand, may have to look a little further than the corner store....
well if you are going to try it i would suggest base with sodium bicarb to prevent a massive increase in pH at the one time, and it will still be able to extract NaBr from the organic crap.... i would reccomend many extractions and cleans afterwards to remove all organic matter....
-AC
Its just my opinion, but no-one listens to me anyway, and rightly so...
(Chief Bee)
08-06-04 09:50
No 523974
Basifying with NaOH to generate an NaBr solution seems potentially like a good idea, unless the molecule does something weird at a high pH.
That might be very hard to do - the compound isn't a hydrobromide salt of an amine, it's a quaternary amine salt and will not release the bromide ions easily.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
08-06-04 12:25
No 523985
Rhodium sorry, but the structure you give in Post 523609 (Rhodium: "Diquat dibromide does not contain bromine", Chemistry Discourse) does not seam to be correct. I saw that structure on this page: http://www.alanwood.net/pesticides/diqua
There are other pages showing a structure that makes more sense, like:
http://www.inchem.org/documents/pds/pds/
Of course, since it is a quartetrnary amine, it is not possible to make NaBr in an easy way from it, but I'm quite sure that the organic part isn't very reactive to Br2. So it should be possible to distil Br2 out of the diquat/H2O2/H2SO4 reaction mixture just like when using KBr or NaBr.
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Chief Bee)
08-06-04 12:31
No 523986
Yes, you are definitely correct - I copied the structure from that page without thinking...
The Hive - Clandestine Chemists Without Borders