(Chief Bee)
01-19-04 11:00
No 483349
(Rated as: good read)
Ueber eine neue Bildungsweise des 1,2-Dimethyl-3-Phenyl-äthylenimins
R. Haberl
Monatsheft 89, 814-816 (1958) (../rhodium/pdf /aziridine2me
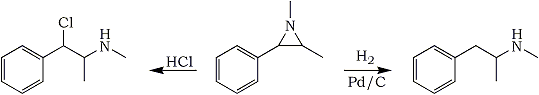
Summary:
The aziridine derived from cyclization of (-)-Ephedrine, 1,2-Dimethyl-3-Phenyl-Aziridine, can be ring-opened by treatment with hydrochloric acid to form (+)-Chloropseudoephedrine (76% yield) or hydrogenated in alcohol with 10% Pd/C at room temp and atmospherical pressure and a 1.5% catalyst load to give (+)-Methamphetamine (89% yield).
The Hive - Clandestine Chemists Without Borders
(Newbee)
01-19-04 11:13
No 483352
That hydrogenation yield and conditions look nice.
Any suggestions on a quick and easy cyclization step
that will form the azirdine in near quantative yield?
Appart from the conventional of course?
I cant believe Im asking this.
Nice one!
(Chief Bee)
01-19-04 13:26
No 483374
(Rated as: good read)
Regio- and stereoselective transformation of trans-1,2,3-trisubstituted aziridines into trans-oxazolidin-2-ones
Luisa Testa, Mohamed Akssira, Elena Zaballos-García, Pau Arroyo, Luis R. Domingo and Jose Sepúlveda-Arques
Tetrahedron 59(5), 677-683 (2003) (../rhodium/pdf /ephedrine-az
DOI:10.1016/S0040-4020(02)01565-X
The preparation of the aziridine is probably most easily done by preparing chloroephedrine (in this article they use SOCl2 - but the classic HCl/ZnCl2 should also work). The crude chloroephedrine is then cyclized by treatment with NaOH.
The Hive - Clandestine Chemists Without Borders
(Wizard Master)
01-20-04 05:05
No 483525
AZIRIDINES FROM â-IODOCARBAMATES
http://www.orgsyn.org/orgsyn/prep.asp?pr
C6H5-CH2-CH=CH2 + I-N=C=O ==> C6H5-CH2-CH(-NHCO2Me)-CHI
http://www.orgsyn.org/orgsyn/prep.asp?pr
Sorry for the link error?
(Hive Bee)
01-20-04 06:48
No 483538
Rhodium Nice article""Ephedrine Aziridine" Reduction to Meth " of what I could decipher, the choice of going either to chloroephedrine or hydrogenating to the meth compound. If I could only figure a friendly way to convert ephedrine to the desired aziridine....
However on the next article "Regio- and stereoselective transformation of trans-1,2,3-trisubstituted aziridines into trans-oxazolidin-2-ones" the choice is now only through chloroephedrine, but then if one's goal is to hydrogenate to meth,, why would one go through chloroephedrine then to the aziridines when one can hydrogenate directly from chloroephedrine....unless there is and advantage of hydrogenating frome aziridines such as higher yields of meth...
WizardX .....the entry,
C6H5-CH2-CH=CH2 + I-N=C=O ==> C6H5-CH2-CH(-NHCO2Me)-CHI
looks interesting ,however unable to locate it after several attempts, how else can I read it ...the fact that I can start from an aryl-alkene to prepare the aziridine to later hydrogenate to meth seems very approachable....java
We're all in this world together,
http://www.aztlan.net
(Miss High & Mighty)
01-20-04 21:28
No 483745
Java,
concider the yield then look at the conditions the hydrogenation is done under.
This is the selling point for me.
It also suggests the possibility of re-cycling those pesky azriridines as aposed to.
(Hive Bee)
01-21-04 08:04
No 483829
ballzofsteel No doubt the yields are much better going from aziridines to meth, however interested in the arylketone route as ephedrine is not available.
WizardX posted a route however not able to locate it, would be nice if someone posted it,no synthesis found on the route by me......java
We're all in this world together,
http://www.aztlan.net
(Wizard Master)
01-21-04 16:10
No 483897
AZIRIDINES FROM â-IODOCARBAMATES
http://www.orgsyn.org/orgsyn/prep.asp?pr
C6H5-CH2-CH=CH2 + I-N=C=O ==> C6H5-CH2-CH(-NHCO2Me)-CHI
http://www.orgsyn.org/orgsyn/prep.asp?pr
(Hive Bee)
01-23-04 17:21
No 484286
Summary of what's known so far,
By the posted referenced synthesis by WizardX we have our general procedure, hence by starting with an arylalkene, for those of us without access to ephedrine,
C6H5-CH2-CH=CH2 -----IN3--> Ref.(a)------
--->C6H5-CH2-CH(NCO)-CH2(I) ----RBCl2/base--->Ref. (b)------
--->C6H5-CH2-CH(NHCOOCH3)-CH2(I) ----KOH/ETOH--> Ref. (b)---------
---->C6H5-CH2-CH-CH2 (1,2-Dimethyl-3-Phenyl-Aziridine)
\ /
N
|
CH3
and by the article ......
Post 483349 (Rhodium: ""Ephedrine Aziridine" Reduction to Meth", Stimulants)
after the catalytic hydrogenation reduction and the conditions outlined in the article posted by Rhodium we can get to the desired product....... methamphetamine ......java
References
1.March 5th ed.pg.1046 "Azido-Iodo addition" 15-43
a.ibid Ref 964 Hassner,A.; Matthews, G.J.; Fowler, F.W. J.Am.Chem.Soc.91,5046, 1969
b.ibid Ref.965Levy, A.B. ; Brown, H.C.J.Am.Chem. Soc.95,4067,1973
2.Cambie, R.C.;Hayward, R.C.; Rutledge, P.S.;Smith-Plamer, T.; Swedlund, B.E. ; Woodgate, P.D. J. Chem. Soc. Perkins Trans.1,180, 1979.
3. Hassner, A.; Keogh, J. J.Org. Chem.51,2767,,1986
4.Azido-Bromo-addition has also been done with another ragent:Olah, G.A.; Wand, Q.; Li,X.; Prakash, G.K.S. Synlet 487,1990
5.Hassner, A.; Keogh,J.Tetrahedral Lett. 1575,1975
6.Hassner, A.; Teeter, J.S.J.Org. Chem.36,2176,1971
7. Even IN3 can be induces to add by a free-radical mechanism (see,ibid ref.#2).For a review of free -radical additions of XN3, see Hassner, A. Intra-Sci. Chem.Rep.4,109,1970
8. Hassner, A.; Isbister, R.J.; Friederang, A.J. Am. Chem. Soc.91,5046,1969
Note. edited by java , thank you WizardX for catching my error 1-24-04
We're all in this world together,
http://www.aztlan.net
(Wizard Master)
01-23-04 21:57
No 484321
Java: Not this... C6H5-CH=CH-CH3
This... C6H5-CH2-CH=CH2
(Hive Bee)
01-25-04 15:25
No 484559
(Rated as: excellent)
by
R. Haberl
Monatsheft 89, 814-816 (1958) (../rhodium/pdf /aziridine2me
(-)-1,2-Dimethyl-3-phenyl-ethylenimin (I) was identified as the by-product of the preparation of (+)-2-phenyl-3,4-dimethylmorpholine by dehydration with concentrated H2SO4 of (-)-N,ß-hydroxyethylephedrin. On hydrogenation as well on treatment with HCl I undergoes ring opening to (+)-1-phenyl-2-methylamino-propan resp. (+)-psi-1-phenyl-1-chlor-2-methylamino-p
The prepration of (+)-2-phenyl-3,4-dimethyl-morpholin from (-)-N-ß-hydroxyethyl-ephedrin via ring closure with concentrated H2O4 according to W. Otto1 can be shown with the following reaction scheme.
Molecule: 1 ("c1(ccccc1)C(C(C)N(C)CCO)O>>C1COC
There is always as a by-product in varying, but always in little yields, a colourless liquid with a b.p.12 85° and a specific rotation of [alpha]18D = -131,7° (alcohol). From the values of the microanalysis and the volatility of the substance there follows the total formula: C10H13N.
The hydrogenation of the compound which already proceeds at room temperature very fast under consumption of an equimolar amount of hydrogen yields an optically active base which hydrochlorid was identified as (+)-1-phenyl-2-methylamino-propan · HCl [(+)-desoxyephedrin · HCl] because the mixed melting point of a (+)-desoxyephedrin · HCl prepared according to H. Emde2 showed no depression and the specific optical rotation of both compounds matched.
On treatment of the by-product in absolute alcohol with absolute alcholic HCl at room temperature until weakly acidic pH a compound was created. The microanalysis gave the the total formula C10H15Cl2N. The product is (+)-psi-1-phenyl-1-chloro-2-methylamino-
By the above experimental results it was shown that the by-product has the structure of 1,2-dimethyl-3-phenyl-ethylenimine (I).
Molecule: 2 ("c1(ccccc1)C2C(C)N2C")
The isomeric phenylpropan with a double bond of I which would behave similiar on hydrogenation and HCl-addition can be excluded as it would be optically inactive.
Compound I was, according to my knowledge, described first by K. Tanaka3 who got it by treatment of (+)-psi-1-phenyl-1-chloro-2-methylamino-
[personal blabla]
Experimental part
1,2-dimethyl-3-phenyl-ethylenimine (I)
When preparing (+)-2-phenyl-3,4-dimethylmorpholine according to W. Otto1 I can be isolated as a lower boiling fraction when distilling the etheral extracts. B.p.12 85°, [alpha]18D = 131,7° (alcohol).
[details of the microanalysis]
Hydrogenation of I to (+)-1-phenyl-2-methylamino-propan
20,0 g I were dissolved in 75 ml alcohol and 0,3 10% Pd/animal charcoal and hydrogenated in a 250 ml Schüttelente [literally: shaker duck, probably a kind of shaken hydrogenation device]. The H2-uptake is very fast at the beginning (2 l in 30 min) and is completed after about 4 hours. H2-consumption: 3110 ml (0°, 760 torr). After filtration of the catalyst vacuum distillation gave 17,9 (+)-1-phenyl-2-methylamino-propan, 88,8% of theory, b.p.12 84°.
(+)-psi-1-Phenyl-1-chloro-2-methylamino-
10,0 g I were dissolved in few absolute alcohol and treated with absolute alcoholic HCl until weakly acidic pH at room temperature. The reaction mixtures was cooled for a few hours with ice and then the formed crystalls were filtered. Yield: 9,5 g, 76% of theory, m.p. 202° (from alcohol), [alpha]18D = 115,2 (water).
[details of the microanalysis]
1. W. G. Otto, Angew. Chem. 68, 181, (1956)
2. H. Emde, Helv. Chim. Acta 12, 365 (1929)
3. K. Tanaka, J. Pharm. Soc. Japan 79, 212 (1950)
The tendency is to push it as far as you can
(Wizard Master)
02-04-04 05:59
No 486329
AZIRIDINES PREPARED BY REDUCTION OF OXIMES WITH LITHIUM ALUMINUM HYDRIDE
http://www.orgsyn.org/orgsyn/prep.asp?pr