09-17-04 04:03
No 531713
National institute on drug abuse "research" states d/l Hydroxylamphetamine is twice as potent as d-meth.Maybe this is the future?So how to add the -OH to amphetamine? Please help.
(Hive Addict)
09-17-04 09:37
No 531806
levo...a very interesting find. I've looked up some leads and found this....
....the reviews mentioned point to the presence of one or more hydroxyl groups in the benzene ring as one of the most potent modifiers of the magnitude of the physiological effects of compounds possessing the basic /3-phenethylamine skeleton.
....as read, Physiologically Active Phenethylamines. I. Hydroxy- and Methoxy-a-methyl-Phenethylamines ( p-Phenylisopropylamines)
E. H. WOODRUFF AND THEODORE W. CONGER
Journal of the American Chemical Society Vol. 60, No. 2, 465,1938
...see Post 531663 (java: "Propenylbenzenes Anyone?", Chemistry Discourse) for the full pdf. Also these are some readings for the OH addition and effects.......
../rhodium
/nitroal
Post 531710 (Rhodium: "N-Hydroxy-Amphetamines from Amphetamines", Novel Discourse)
which may help to see the mechanism for adding the OH to an amine. I will keep this in mind, as I do my daily run through the chemistry archives on the web.
Would be interested in following your quest , so feel free to report it on this thread.....java
Note: edited by java
It is better to die on your feet than to live on your knees...Emiliano Zapata
(Chief Bee)
09-18-04 11:22
No 532013
levo: Could you provide us with a reference to what NIDA publication states that racemic N-OH-amphetamine would be of higher potency than d-methamphetamine? I would bet the opposite was true in regards to CNS stimulation.
The Hive - Clandestine Chemists Without Borders
(Stranger)
09-18-04 21:05
No 532089
For information on availability of NIDA RESEARCH MONOGRAMS 1-24(1975-1979),write to NIDA Office for Research Communications,Room 10A-54,5600 Fishers Lane,Rockville,MD 20857.....also see....NITIS(national technical information service)U.S. department of Commerce Springfeild,VA 22161....GPOsuperintent of Documents US government printing office Washington,DC 20402-REF-Neuropharmacolgy 23:803-806,1984...REF-Biochem Pharmacol 22:311-322,1973....REF-J Pharm Pharmacol 24:171-173,1972.....REF-Eur J Pharmacol 148:195,1988.
(Chief Bee)
09-18-04 21:31
No 532093
Here is the list of the NIDA monographs (online): http://www.drugabuse.gov/pdf/monographs/
Which one is it, and on what page?
The Hive - Clandestine Chemists Without Borders
(Stranger)
09-19-04 10:14
No 532173
Here it is let me know if I read it correctly.....Pharmacology&Toxicology of amphetamines&Related Designer Drugs-#94(1989)pages-50&51 STIMULUS PROPERTIES....well maybe not twice as potent as d-meth but never the less its still stronger...but it is twice as stronge as AMPS best
(Hive Addict)
09-19-04 15:00
No 532214
Upon reading the said study it shows it takes less than half of the dose as that of amph for the effect in the study....
....The N-hydroxy analog of AMPH (i.e., N-OH AMPH), a metabolite of AMPH, also produces AMPH-like effects and is about twice as potent as AMPH.
So I guess putting the OH of the amine will boost the effect to twice that of amph making almost as potent as methamphetamine......now which is easier to do starting with amph, seems like methylation would be easier.............as I see it, java
It is better to die on your feet than to live on your knees...Emiliano Zapata
(Chief Bee)
09-19-04 15:27
No 532218
From http://www.drugabuse.gov/pdf/monographs/
"The N-hydroxy analog of AMPH (i.e., N-OH AMPH), a metabolite of AMPH, also produces AMPH-like effects and is about twice as potent as AMPH (table 2)."
From Table 2:
dl-N-Hydroxyamphetamine 1.1 Ámole/kgThis shows that both dl-N-Hydroxyamphetamine and d-Methamphetamine are about twice as potent as dl-Amphetamine. The d-isomer of the hydroxy analog may be more potent than d-Methamphetamine, but it is certainly of a shorter duration as N-hydroxy-amines are metabolized rather rapidly. I believe that nothing can be predicted about the potency of N-hydroxy-methamphetamine, as it is a tertiary amine, and they are in general of rather low potency, as can be seen with N,N-dimethylamphetamine and N,N-dimethylcathinone. However, as hydroxy groups are not methyl groups, nothing can be predicted with certainity.
dl-Amphetamine 2.6 Ámole/kg
dl-Methamphetamine 1.5 Ámole/kg
d-Methamphetamine 1.2 Ámole/kg
The Hive - Clandestine Chemists Without Borders
(Hive Addict)
09-19-04 15:58
No 532227
I don't have the time to look this up now, but isn't this substance used in some eye therapy? Therefore all the pharmacokinetics et cetera should be findable.

(Stranger)
09-20-04 06:03
No 532324
In the good book let's turn to pages 671,682,683,684,&685....OPPS...sorry...t
(Hive Addict)
09-20-04 16:21
No 532392
The question then begs to be asked , how will methamphetamine behave with a hydroxyl attached along side the amine,
C6H5-CH2-CH(NOH-CH3)-CH3
I think this is right, anyway just a question that I guess was on my mind ........java
It is better to die on your feet than to live on your knees...Emiliano Zapata
(Hive Martyr)
09-25-04 07:39
No 533078
You know when WizardX busts out with a whole paragraph of technical chemistry jargon.. .that the subject has been covered.
I dont know what that shit means, but covered sounds good.
http://www.dodgeit.com
(Stranger)
09-25-04 09:01
No 533090
He just told us the method for obtaining the P2P oxime that we've been talking about that makes it pointless to combine P2P with hydroxyamine. WizardX, can you fish out a method to make d-hydroxyamphetamine?
(Stranger)
09-25-04 18:35
No 533139
Here's two sites I got from the king bee......./rhodium/pdf /n-oh-amphetam
(Hive Addict)
09-25-04 20:07
No 533148
(Rated as: excellent)
levo....you should read the entries on your thread as my first reply to your inquiry I posted Rhodium's articles Post 531806 (java: "How to get to Hydroxylamphetamine.....", Stimulants) and had you read, this is what you missed.....
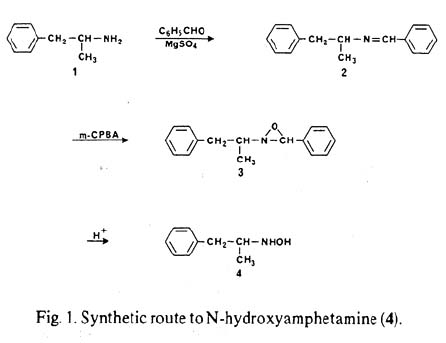
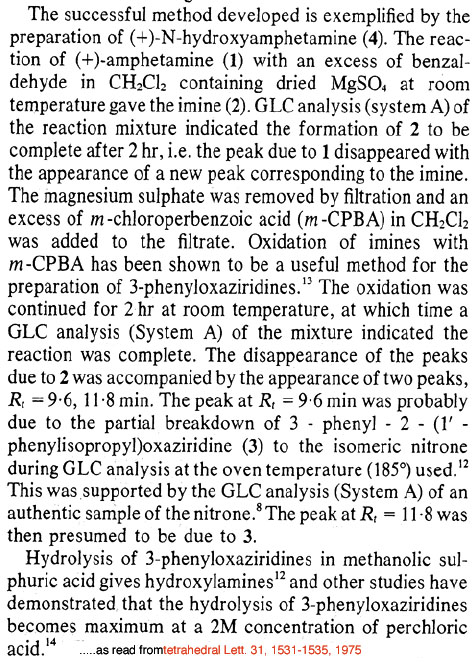
and by the way the link you provided doesn't seem work .......java
Note...edited by java levo the articles you referenced are at Post 531710 (Rhodium: "N-Hydroxy-Amphetamines from Amphetamines", Novel Discourse) as mentioned in my first post
It is better to die on your feet than to live on your knees...Emiliano Zapata
(Stranger)
09-26-04 00:48
No 533177
Well bless your hard pumping heart!!!!I know the link doesn't work-its written just like I got it.I've asked Rhodium to look at the problem-check back later.
(Chief Bee)
09-27-04 15:12
No 533380
See Post 531710 (Rhodium: "N-Hydroxy-Amphetamines from Amphetamines", Novel Discourse) for the correct links.
The Hive - Clandestine Chemists Without Borders
(Hive Addict)
10-12-04 22:17
No 535609
N-Oxygenation of Amphetamine and Methamphetamine by the Human Flavin-Containing Monooxygenase (Form 3): Role in Bioactivation and Detoxication1
John R. Cashman2 , Yeng N. Xiong, Lifen Xu and Aaron Janowsky2
Pharmacology (1999) Vol. 288, Issue 3, 1251-1260
http://jpet.aspetjournals.org/cgi/conten
Abstract
(+)- And ()-amphetamine and methamphetamine were N-oxygenated by the cDNA expressed adult human flavin-containing monooxygenase form 3 (FMO3), their corresponding hydroxylamines. Two major polymorphic forms of human FMO3 were studied, and the results suggested preferential N-oxygenation by only one of the two enzymes. Chemically synthesized (▒)-amphetamine hydroxylamine was also a substrate for the human FMO3 and it was converted to phenylpropanone oxime with a stereoselectivity ratio of trans/cis of 5:1. Human FMO3 also N-oxygenated methamphetamine to produce methamphetamine hydroxylamine. Methamphetamine hydroxylamine was also N-oxygenated by human FMO3, and the ultimate product observed was phenylpropanone. For amphetamine hydroxylamine, studies of the biochemical mechanism of product formation were consistent with the production of an N,N-dioxygenated intermediate that lead to phenylpropanone oxime. This was supported by the observation that -deutero (▒)-amphetamine hydroxylamine gave an inverse kinetic isotope effect on product formation in the presence of human FMO3. For methamphetamine, the data were consistent with a mechanism of human FMO3-mediated N,N-dioxygenation but the immediate product, a nitrone, rapidly hydrolyzed to phenylpropanone. The pharmacological activity of amphetamine hydroxylamine, phenylpropanone oxime, and methamphetamine hydroxylamine were examined for effects at the human dopamine, serotonin, and norepinephrine transporters. Amphetamine hydroxylamine and methamphetamine hydroxylamine were apparent substrates for the human biogenic amine transporters but phenylpropanone oxime was not. Presumably, phenylpropanone oxime or nitrone formation from amphetamine and methamphetamine, respectively, represents a detoxication process. Because of the potential toxic nature of amphetamine hydroxylamine and methamphetamine hydroxylamine metabolites and the polymorphic nature of N-oxygenation, human FMO3-mediated metabolism of amphetamine or methamphetamine may have clinical consequences.
It is better to die on your feet than to live on your knees...Emiliano Zapata
(Hive Addict)
10-13-04 07:17
No 535609
(Rated as: good read)
N-Oxygenation of Amphetamine and Methamphetamine by the Human Flavin-Containing Monooxygenase (Form 3): Role in Bioactivation and Detoxication1
John R. Cashman2 , Yeng N. Xiong, Lifen Xu and Aaron Janowsky2
Pharmacology (1999) Vol. 288, Issue 3, 1251-1260
http://jpet.aspetjournals.org/cgi/conten
Abstract
(+)- And ()-amphetamine and methamphetamine were N-oxygenated by the cDNA expressed adult human flavin-containing monooxygenase form 3 (FMO3), their corresponding hydroxylamines. Two major polymorphic forms of human FMO3 were studied, and the results suggested preferential N-oxygenation by only one of the two enzymes. Chemically synthesized (▒)-amphetamine hydroxylamine was also a substrate for the human FMO3 and it was converted to phenylpropanone oxime with a stereoselectivity ratio of trans/cis of 5:1. Human FMO3 also N-oxygenated methamphetamine to produce methamphetamine hydroxylamine. Methamphetamine hydroxylamine was also N-oxygenated by human FMO3, and the ultimate product observed was phenylpropanone. For amphetamine hydroxylamine, studies of the biochemical mechanism of product formation were consistent with the production of an N,N-dioxygenated intermediate that lead to phenylpropanone oxime. This was supported by the observation that -deutero (▒)-amphetamine hydroxylamine gave an inverse kinetic isotope effect on product formation in the presence of human FMO3. For methamphetamine, the data were consistent with a mechanism of human FMO3-mediated N,N-dioxygenation but the immediate product, a nitrone, rapidly hydrolyzed to phenylpropanone. The pharmacological activity of amphetamine hydroxylamine, phenylpropanone oxime, and methamphetamine hydroxylamine were examined for effects at the human dopamine, serotonin, and norepinephrine transporters. Amphetamine hydroxylamine and methamphetamine hydroxylamine were apparent substrates for the human biogenic amine transporters but phenylpropanone oxime was not. Presumably, phenylpropanone oxime or nitrone formation from amphetamine and methamphetamine, respectively, represents a detoxication process. Because of the potential toxic nature of amphetamine hydroxylamine and methamphetamine hydroxylamine metabolites and the polymorphic nature of N-oxygenation, human FMO3-mediated metabolism of amphetamine or methamphetamine may have clinical consequences.
It is better to die on your feet than to live on your knees...Emiliano Zapata