(Chief Bee)
06-20-03 15:22
No 441399
(Rated as: excellent)
The Oxidation of Primary Alcohols to Methyl Esters and Diols to Lactones Using Trichloroisocyanuric Acid
Gene A. Hiegel; Cynthia B. Gilley
Synthetic Communications 33(12), 2003-2009 (2003) (../rhodium/pdf /bdo2gbl.tric
DOI:10.1081/SCC-120021026
Abstract
Primary alcohols and diols are easily oxidized to methyl esters by a solution of trichloroisocyanuric acid with methyl alcohol in dichloromethane. In addition,
 ,
, -diols are also readily oxidized into lactones by refluxing with trichloroisocyanuric acid and pyridine in dichloromethane.
-diols are also readily oxidized into lactones by refluxing with trichloroisocyanuric acid and pyridine in dichloromethane.Although a variety of oxidizing agents have been shown to successfully convert diols to lactones, (1–8) there are few methods for the synthesis of methyl esters from primary alcohols. N-Iodosuccinimide, (7) calcium hypochlorite, (9) and tert-butyl hypochlorite (10) have been shown to oxidize primary alcohols to methyl esters. We wish to report simple procedures for the synthesis of methyl esters from primary alcohols and for lactones from diols using trichloroisocyanuric acid (1) [1,3,5-trichloro-1,3,5- triazine-2,4,6-(1H,3H,5H)-trione] as the oxidizing agent. (11)
Primary alcohols and diols are easily converted into the corresponding methyl esters in a solution of 1 and methyl alcohol in dichloromethane (Eq. (1 )). The yield and purity of the isolated methyl esters are shown in Table 1 .
Equation 1
3 ROH + 2 C3N3O3Cl3 + 3 CH3OH
 3 RCO2CH3 + 2 C3H3N3O3 + 3 HCl
3 RCO2CH3 + 2 C3H3N3O3 + 3 HClA reasonable pathway for the transformation is shown in the Sch. 1 . Previous studies have shown that the aldehyde to methyl ester conversion (12) is significantly faster than the conversion of primary alcohols to aldehydes. (13)
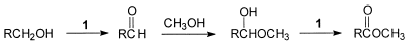
Scheme 1
The primary alcohol to methyl ester conversion is an exothermic reaction, but an increase in reaction temperature results in the formation of byproducts. In addition, there is a variable induction period between the time the reagents are mixed and when the temperature spikes. Characteristically cyanuric acid begins to precipitate as the temperature begins to rise. The oxidation of primary alcohols to methyl esters using 1 was carried out successfully with methyl alcohol in dichloromethane, with methyl alcohol in acetonitrile, and in methyl alcohol. The solvent of choice for moderate or large-scale reactions is dichloromethane because the temperature is controlled internally by the low boiling point. If the temperature is kept at or below 40°C, methanol or acetonitrile can be used as solvents. Since external heat control can be problematic, the recommendation is to use dichloromethane as the solvent and to place the reaction flask in a cold water bath to facilitate heat dissipation. Since chlorine is also present in the reaction mixture, the reaction was protected from light to prevent radical chain chlorination reactions.
A complex mixture of products was formed during the oxidation of phenethyl alcohol and 3-phenyl-1-propanol. Once phenethyl alcohol is oxidized to phenylacetaldehyde, it is expected to have a higher enol content than most aldehydes, and therefore, more likely to form the
 -chloro derivative. Other byproducts may also result from chlorination on the aromatic ring. (11)
-chloro derivative. Other byproducts may also result from chlorination on the aromatic ring. (11) The C4 and C5
 ,
, -diols can be converted into their corresponding lactones by refluxing with 1 and pyridine in dichloromethane. The yield and purity of the isolated lactones are shown in Table 2 . Pyridine is added to absorb the hydrogen chloride formed during the oxidation. Experiments performed without pyridine gave low yields of lactone possibly due to polymer formation. The lactone formation reaction is believed to proceed through a cyclic hemiacetal, which would then be oxidized to the lactone.
-diols can be converted into their corresponding lactones by refluxing with 1 and pyridine in dichloromethane. The yield and purity of the isolated lactones are shown in Table 2 . Pyridine is added to absorb the hydrogen chloride formed during the oxidation. Experiments performed without pyridine gave low yields of lactone possibly due to polymer formation. The lactone formation reaction is believed to proceed through a cyclic hemiacetal, which would then be oxidized to the lactone. In contrast, the oxidation of 1,6-hexanediol gave only a trace amount of -caprolactone. However, 1,4-butanediol, 1,5-pentanediol, 1,6-hexanediol and 1,12-dodecanediol did oxidize to the corresponding diesters when methyl alcohol was present. The dimethyl succinate from the oxidation of 1,4-butanediol contained an unidentified impurity of 11.3% which may be 2-methoxytetrahydrofuran based on an 1H-NMR peak at 3.23
 .
.Table 1 The oxidation of alcohols to methyl esters.
Yields 75-96%, Except 1,4-butanediol (39%) and 1,5-pentanediol (67%)
Table 2 The oxidation of
 ,
, -diols to lactones.
-diols to lactones. | Butanediol | Butyrolactone | 76% | 98.4% |
| Pentanediol | Valerolactone | 72% | 97.4% |
 , , -diol -diol |
Lactone | Yield | Purity (GC) |
Experimental
All reagents were used as received unless otherwise stated. TCICA was obtained from OMNI with a purity of 99% (88% available chlorine). All products were purified by distillation or flash chromatography and were characterized by comparison with authentic samples using IR and 1H-NMR spectra and GC retention time.
Oxidation of Benzyl Alcohol to Methyl Benzoate
In a 250-mL three-neck flask were placed a magnetic stir bar, 60 mL of anhydrous dichloromethane, 39.0 mL (963 mmol) of anhydrous methyl alcohol, and 22.46 g (96.6 mmol) of 1. After approximately 10 min of stirring, 10.28 g (10 mL, 95.1 mmol) of benzyl alcohol was added. The flask was flushed with nitrogen, covered with foil to keep out light, and placed in a cold-water bath (2°C at start of the reaction). After approximately 24 h, excess TCICA was destroyed by the slow addition of saturated aqueous sodium hydrogen sulfite until a negative test with wet potassium iodide-starch test paper was achieved. The cyanuric acid precipitate was removed by vacuum filtration and the solid was washed with pentane. Most of the solvent was removed from the filtrate with a rotary evaporator and the residue was diluted with 60 mL of pentane. The pentane solution was washed with 1 N NaOH (20 mL), saturated NaCl (20 mL), and dried over anhydrous magnesium sulfate. After filtration and concentration, the crude product was vacuum distilled [b.p. 96.0–97.8°C (26.4–30.3 torr)] through a concentric tube column to give 10.16 g (79%) of methyl benzoate. Analysis by GC showed a purity of 99.6%, and the retention time was identical to that of an authentic sample. The IR and 1H-NMR spectra were identical to that of an authentic sample.
Oxidation of 1,4-Butanediol to
 -Butyrolactone
-ButyrolactoneIn a 50-mL three-neck flask were placed a magnetic stir bar, 30.0 mL of anhydrous dichloromethane, 0.4544 g (5.042 mmol) of 1,4-butanediol, 0.90 mL (11.2 mmol) of pyridine, and 1.2899 g (5.550 mmol) of 1. The flask was flushed with nitrogen, covered with foil to keep out light, and refluxed using a temperature-controlled oil bath. After approximately 5 h, excess TCICA was destroyed by the slow addition of saturated aqueous sodium hydrogen sulfite until a negative test with wet potassium iodide-starch test paper was achieved. The cyanuric acid precipitate was removed by vacuum filtration and the solid was washed with dichloromethane. The solution was washed with 1 N HCl (15 mL). Dichloromethane (2 × 15 mL) was used to back-extract the HCl wash. The solution was then dried over anhydrous sodium sulfate. After filtration and concentration, the oily residue was purified by flash chromatography to give 0.3305 g (76%) of
 -butyrolactone. Analysis by GC showed a purity of 98.4%, and the retention time was identical to that of an authentic sample. The IR and 1H-NMR spectra were identical to that of an authentic sample.
-butyrolactone. Analysis by GC showed a purity of 98.4%, and the retention time was identical to that of an authentic sample. The IR and 1H-NMR spectra were identical to that of an authentic sample.References
[1] Shaabani A., Lee D.G., Solvent free permanganate oxidations,
Tetrahedron Lett., 42 (34), (2001) 5833–5836. (../rhodium/pdf /solventfree.
[2] Tanaka H., Kawakami Y., Goto K., Kuroboshi M., An aqueous silica gel disperse electrolysis system. N-Oxyl-mediation electrooxidation of alcohols,
Tetrahedron Lett., 42 (3), (2001) 445–488. Post 495126 (Rhodium: "1,4-BD -> GBL (4.5h, 84%) Electrooxidation", Methods Discourse)
[3] Mukhopadhyay S., Chandalia S.B., Production of
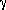 -butyrolactone by liquid phase oxidation of 1,4-butanediol,
-butyrolactone by liquid phase oxidation of 1,4-butanediol,Indian J. Chem. Technol., 6 (4) , (1999) 237–239. Post 444358 (Rhodium: "BDO to GBL w/ NaOCl/RuCl3 (Conv 83%, Select 97%)", Methods Discourse)
[4] Yang G., Chen Z., Zhang S., Chen J., Study on polymer-supported tribromide oxidizing agent,
Lizi. Jiaohuan Yu Xifu, 14 (6) , (1998) 475–480. Post 441437 (Rhodium: "Chem. Abs. 130:24638.", Methods Discourse)
[5] Yang G., Chen Z., Shi C., Study on polymer-supported bromate ion oxidizer with sodium bisulfate,
Hubei Daxue Xuebao, Ziran Kexueban., 20 (3) , (1998) 256–259.
Post 441437 (Rhodium: "Chem. Abs. 130:24638.", Methods Discourse)
[6] Morimoto T., Hirano M., Iwasaki K., Ishikawa T., Oxidation of diols with sodium bromite trihydrate in organic solvents in the presence of alumina,
Chem. Lett., (1) , (1994) 53–54. Post 442866 (Rhodium: "1,4-BD -> GBL (92%) using NaBrO2/Alumina", Methods Discourse) Post 443378 (Vitus_Verdegast: "article typed up", Methods Discourse)
[7] McDonald C.E., Holcomb H.L., Leathers T.W., Kennedy K.E., The oxidation of primary alcohols to methyl esters and lactones using N-iodosuccinimide,
Microchem. J., 47 (1–2) , (1993) 115–119.
[8] Kondo S., Kawasoe S., Kunisada H., Yuki Y., Convenient synthesis of lactones by the reaction of diols with N-halomides,
Synth. Commun., 25 (1995) 719–724. two examples using 1 are included. Post 500826 (Rhodium: "GBL from 1,4-Butanediol using N-Haloamides", Methods Discourse)
[9] McDonald C.E., Nice L.E., Shaw A.W., Nestor N.B., Calcium hypochlorite-mediated oxidation of primary alcohols to methyl esters,
Tetrahedron Lett., 34 (17) , (1993) 2741–2744. (../rhodium/pdf /alcohols2met
[10] Milovanovic J.N., Vasojevic M., Gojkovic S., Oxidation of primary alcohols to methyl esters using tert-butyl hypochlorite, pyridine and methyl alcohol,
J. Chem. Soc. Perkin Trans. 2, (1991) 1231–1233.
[11] Hiegel G.A. Trichloroisocyanuric Acid, Encyclopedia of Reagents for Organic Synthesis, Paquette L.A. Wiley, Chichester, England, 1995, Vol. 7 pp. 5072–5073. For synthetic applications of 1 see:
[12] Hiegel G.A., Bayne C.D., Donde Y., Tarmashiro G.S., Hilberath L.A., The oxidation of aldehydes to methyl esters uisng trichloroisocyanuric acid,
Synth. Commun., 26 (1996) 2633–2639.
[13] Unpublished results by Katherine A. Alvarado and Klaas Schildknegt, The oxidation of primary alcohols with trichloroisocyanuric acid.
(Chief Bee)
06-20-03 17:30
No 441437
Study on polymer-supported bromate ion oxidizer with sodium bisulfate
Yang, Guichan; Chen, Zuxing; Shi, Congyun
Hubei Daxue Xuebao, Ziran Kexueban 20(3), 256-259 (1998)
ISSN: 1000-2375 - CAN 130:24638
Abstract
Polymer-supported bromate ion oxidizer was prepared from strong basic ion-exchange resin with sodium bromate. The primary alcohols and simple ethers were effectly oxidized to esters, secondary alcohols to ketones,
 ,
, -diols and cyclic ether to lactone, thiol and selenol to disulfide and diselenide in the presence of sodium bisulfite with polymer-supported bromate ion oxidizer. Higher yields were obtained.
-diols and cyclic ether to lactone, thiol and selenol to disulfide and diselenide in the presence of sodium bisulfite with polymer-supported bromate ion oxidizer. Higher yields were obtained.
(Chief Bee)
06-20-03 17:32
No 441438
Study on polymer-supported tribromide oxidizing agent
Yang, Guichun; Chen, Zuxing; Zhang, Shengli; Chen, Jiawei
Lizi Jiaohuan Yu Xifu (1998), 14(6), 475-480.
ISSN: 1001-5493 - CAN 130:313445
Abstract
Polymer-supported tribromide oxidizing agent was prepared by 717# strong base ion exchange resin and elemental bromine. The quantity of the polymer-supported tribromide oxidizing agent was detd. Primary alcohols and simple ethers were oxidized to esters, benzyl alcohol to benzaldehyde, second alcohols to ketones, and
 ,
, -diols and cyclic ethers to lactones by the polymer-supported tribromide oxidizing agent. The oxidized products were confirmed by IR and 1H-NMR, and good yields were obtained.
-diols and cyclic ethers to lactones by the polymer-supported tribromide oxidizing agent. The oxidized products were confirmed by IR and 1H-NMR, and good yields were obtained.
(Chief Bee)
06-22-03 20:33
No 441834
Tetrahedron Letters, 22(41), 4073-4076 (1981) (../rhodium/pdf /bd2gbl.cuo.p
Could we please have this translated to English?
(Hive Addict)
06-23-03 02:00
No 441898
(Rated as: excellent)
Tsss... I never expected my one-year stay in France would have some advantages language-wise, so let's give it a shot. However, I hope that the French I needed every day ("Nique salope, nique!" and "Suçoter, mèrde, déglutir!") does not interfere with the translation of this scientifically written paper.
The synthesis of esters by dehydrogenation of primary alcohols in the gaseous phase catalyzed by CuO - a prelimenary report
Bruno BERTHON, Alain FORESTIERE, G LELEU, B SILLION
Tetrahedron Letters 22(41) (1981) 4073-4076
Abstract - Linear primary alcohols with at least 7 C atoms are quite quantitatively transformed in esters, by CuO, at temperatures > 170°C, without air in liquid phase. Preponderant influence of C in position 2 is evidenced. In the same conditions lactones are obtained from diols, and benzylic alcohols undergoes, by hydride transfer, a disproportionation into toluene, benzaldehyde and water.
Article - The study of the dehydrogenation of primary alcohols with Cu-based catalysts has never stopped since the achievements made by Sabatier and Senderens [1]. It is already well-known that lower primary alcohols form esters when passed through the vapour phase over Cu, but the reaction shows poor selectivity [2,3 and references cited therein]. In case of heavy primary alcohols (having 5 C atoms and more), vapour phase dehydrogenation at 250-300°C over CuO yields the corresponding aldehydes selectively [4].
We now found that a primary alcohol with at least 7 C-atoms yields esters in a higher yield, when the reaction mixture is heated to 170°C in the liquid phase and in the presence of catalytic amounts of CuO. The followed reaction is:
The applied procedure is as follows: an RB flask is equipped with a Dean-Stark apparatus, and is charged with the alcohol and CuO (1% of the alcohol's weight). The mixture is heated and stirred at more than 170°C. The reaction is followed by GC and the reaction mixture is distilled. The reaction can only be performed in water and air free conditions. This necessitates the removal of the minute amount of water formed in the beginning of the reaction. Two observations should be communicated: the formation of metallic copper during the reaction is noted, and when it is stirred in the presence of air, the colorless reaction mass turns blue after filtration.
Results are presented in the following table and give an impression of the limitations of the applied procedure.
| R-CH2OH | reaction temperature | time duration (h) | mol% CuO | % conversion | yield of ester or lactone (c) and (e) | other products (yield) | |
| 1 | CH3(CH2)5 | 180°C | 20 | 0.014 | 100 | 91 | tridecanone-7 (4%, a) |
| 2 | CH3(CH2)8 | 230°C | 23 | 0.019 | 100 | 92 | nonadecanone-10 (trace, a) |
| 3 | CH3(CH2)10 | 260°C | 5 | 0.023 | 100 | 90 | trieicosanone-12 (10%, a) |
| 5 | HO-CH2- | 200°C | 20 | 0.060 | - | - | starting product |
| 7 | HO-(CH2)4- | 230°C | 10 | 0.012 | 85 | 41 (d) | polyesters |
| 8 | HO-(CH2)5- | 230°C | 10 | 0.013 | 85 | 5 | polyesters |
(a) Structural elucidation was performed via MS and/or IR, 1H NMR, elementary analysis and - in case of ketones - also via the dinitro-2,4-phenylhydrazone.
(b) The statistical composition of the mixture is determined via GC of the reaction products, both normal and saponified products.
(d) Valerolactone is partially obtained by depolymerization during the distillation process.
(e) Yield are calculated via GC.
The only demonstrable secondary reaction product when working with non-branched primary alcohols (cf 1-4), is the symetrical ketone formed by decomposition with setting free CO. This gas is detected via GC next to H2, which is formed during the main reaction.
When analyzing the formation of reaction products in function of time [6], the presence of aldehydes is noted in the beginning of the reaction. These aldehydes, however, disappear after a few hours (cf not-show figure; refer to original text s.v.p.). A closer study of this graph demonstrates among other things that the ketone is formed from the beginning of the reaction, seemingly by a concurring process.
Substitution of C2 decreases or inhibits reactivity. That is why in case of substitution by an alcoyl group (cf 9), the formation of aldehyde in a vast quantity next to the ester is observed; when substituting with a C2-disubstituted product (cf 10), the reaction is completely inhibited. Similar remarks have to be made for substituents with an oxygen atom (cf 5, 11, 12).
In case of diols 1-4, 1-5 and 1-6 (cf 6, 7 and 8), total conversion is obtained. The reaction evolves via the formation of lactone and/or polyesters, since the obtained lactones are prone to polymerization [7]. Concerning benzyl alcohol: the formation of ester is not observed, but equimolecular quantities of benzaldehyde, toluene and water are to be found. Kawamoto and Nishimura [2] have made similar observations when they passed phenyl-1-ethanol in the gas phase over Cu and obtained equimolecular yields of acetophenone and ethylbenzene. However, we found that reaction of o-deuterated benzyl alcohol selectively leads to benzaldehyde and non-deuterated toluene with high selectivity. All deuterium is to be found in the formed water, which means that a hydride-tranfering mechanism forms the basis of this reaction.
Kinetic and reaction mechanism studies will follow.
References
cf original text: ../rhodium/pdf /bd2gbl.cuo.pd
The faster you run, the quicker you die.
(Chief Bee)
06-27-03 02:20
No 442866
Oxidation of 1,4-Butanediol to gamma-Butyrolactone using Sodium Bromite Trihydrate and Alumina
Chemistry Letters, pp 53-54 (1994) (../rhodium/pdf /bdo2gbl.brom
NaBrO2.3H2O/Alumina oxidation of 1,4-Butanediol to gamma-Butyrolactone in 92% yield. (Could somebody type the article and post in this thread, please?)
(Hive Bee)
06-29-03 16:46
No 443378
(Rated as: excellent)
CHEMISTRY LETTERS pp. 53-54, 1994
Oxidation of diols with Sodium Bromite Trihydrate in Organic Solvents in the presence of Alumina
Takashi MORIMOTO, Masao HIRANO, Keiko IWASAKI, and Takashi ISHIKAWA
Sodium bromite trihydrate 1 (referred to simply as NaBrO2) is a versatile reagent for the oxidative transformation of many classes of organic compounds1 inclusive of diols2 in aqueous media, but its behavior in a water-free solvent has not been fully studied3. In the course of the work aiming at the oxidation of various functional groups with 1 in aprotic solvents, succesful conversion of diols to the corresponding lactones and/or the hydroxy ketones was found in dichloromethane (DM) or acetonitrile (AN) with the aid of alumina under mild conditions.4
Thus various symmetrical 1,4- and 1,5-diols were treated with the NaBrO2/alumina system in DM at room temperature under anaerobic conditions, giving the five- and six-membered lactones, respectively, in fair yields; the following examples are representative.
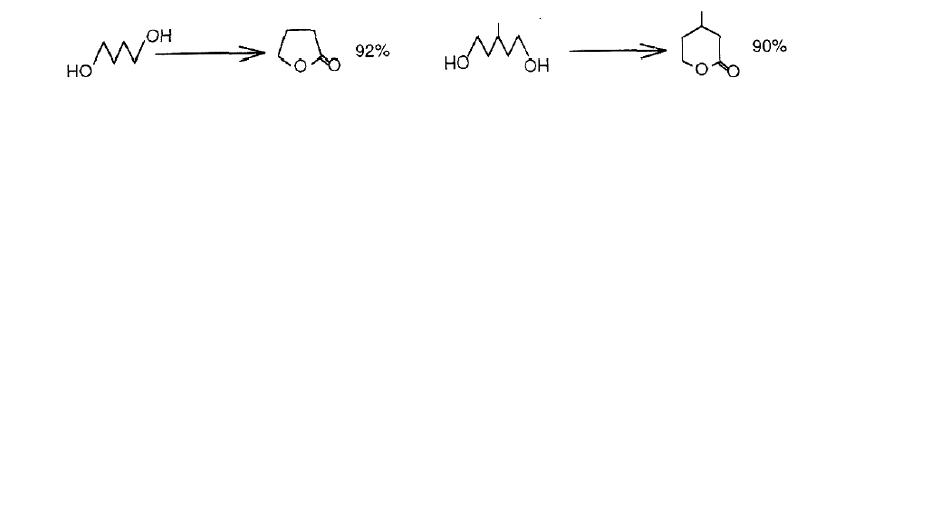
The oxidation of unsymmetrical 1,4- and 1,5-diols did not give the hydroxy ketones, but "abnormally" led to lactones (Table 1): use of AN instead of DM allows the reaction time of a diol with a long alkyl-chain to be lessened considerably without affecting the course of the reaction. This unexpected lactone formation is of special interest, because this phenomenon is markedly contrast to that observed in the conventional oxidation in aqueous medium2, in which secondary hydroxy group was "normally" oxidized in preference to primary one, giving the hydroxy ketone exclusively. Hence, the oxidation of various types of diols was then attempted.
The oxidation of 1,2-diols gave the hydroxy ketones without concurrent carbon-carbon fission as shown above, while the lactones were absent. Similarly, selective formation of the hydroxy ketones from 1,3-diols was observed as typically exemplified by butane-1,3-diol (vide supra). On the other hand, neither a primary nor a secondary hydroxy group was selectively oxidized in the case of 1,6-diols; viz. hexane-1,6-diol gave a complex mixture containing the corresponding epsilon-lactone (max. 38% by GLC) and heptane-1,6-diol gave both 2-methyl-epsilon-lactone (56%) and the hydroxy ketone (44%). Thus, these diols showed the intermediate properties between those of 1,4- and 1,5-diols and 1,2- and 1,3-diols.
Although mechanistic approach is beyond this article, the varying results with the types of diols, especially the tabulated lactone formation, appears to be related to heterogeneous milieu, that is, to the stereochemistry of an absorbed diol on the alumina surface, since 1 is insoluble in DM and no perceptible reaction takes place without alumina. Accordingly, alumina plays important roles not only in increasing the active surface of a given amount of 1, but in determining reactivity of a diol and selectivity of a product.
References.
1. T. Kageyama, Y. Ueno and M. Okawara, Synthesis, 1981, 815;
M. Okawara, Yuki Gosei Kagaku Kyokai Shi. 42, 751 (1984)
2. T. Kageyama, S. Kuwahara, K. Kitahara, Y. Ueno and M. Okawara, Chem. Lett. 1983, 1097
3. T. Kageyama and T. Yamamoto, Chem. Lett., 1980, 671;
idem. Makromol. Chem. 182, 705 (1981)
4. The present oxidation procedure using commercial dry alumina (ICN BIOCHEMICALS Alumina A. Super I) is very simple and almost the same as that shown previously; M. Hirano, M. Oose, and T. Morimoto, Chem. Lett., 1991, 331;
idem. Bull. Soc. Chim. Jpn. 64, 1046 (1991)
http://hes.iki.fi/video/LSD_Being_Tested
(Active Asperger Archivist)
07-02-03 08:47
No 444004
(Rated as: excellent)
Direct Conversion of Ethers to Esters by Trichloroisocyanuric Acid
Tetrahedron Letters No. 55, pp. 5819-5820, (1968)
Abstract:
Use of said title reagent to oxidize alkyl ethers into the corresponding alkyl esters.
The direct oxidation of ethers of formula R-CH2-O-R’ has been reported1 to give alcohols, acids and carboxylates when effected with molecular oxygen. There seems to be no general laboratory method for conversion of ethers to esters in good yield. We are unable to find any report of the use of N-halogen compounds or hypohalites for direct conversion of diethyl ether or other aliphatic ethers to esters. Benzyl ethers have been reported to undergo oxidative cleavage to yield benzaldehydes on treatment with NBS and other oxidizing reagents.2
We wish to report the production of carboxylic acid esters directly from ethers of formula R-CH2-O-R’ by the use of trichloroisocyanuric acid (1) in the presence of an excess of water.
Table 1
| R | R’ | Reaction Conditions (3*C)3 | Major product and Yield4 | Minor Products |
| CH3 | C2H5 | Stir 3 hr, excess Et2O | CH3COOC2H5, 49% | None |
| n-C3H7 | n-C4H9 | Stir 20 hr, excess Bu2O5 | C3H7COOC4H9, 100% | None |
| C6H5 | C2H5 | Stir 15 hr, sl. excess of substrate | Benzaldehyde, 53%6 | Ethyl Benzoate, 4.7% |
Table 1 shows the results of some preliminary work. All reactions were run in the presence of a quantity of water equal to or slightly greater than three times the molar amount of (1) present. Thus, if 0.05moles of (1) were used, 0.15-0.20 moles of water were included in the reaction mixture.
In another experiment, it was found we were able to convert benzyl alcohol to benzaldehyde in 53.7% by reaction with trichloroisocyanuric acid in the presence of an excess of water. It is noted in Table 1 that benzyl ether produced benzaldehyde as its major product. It is interesting that this reagent (i.e. trichloroisocyanuric acid), which generates esters from ethers, will, under the same conditions, oxidize benzyl alcohol no further than the aldehyde.
References:
1.a. H. Grimm and W. Flemming (to I.G. Farbenind A.G.), Ger. 728, 384, November 5, (1942). Chem. Abs. 38, p379 (1944).
1.b. V.I. Stenberg, R.D. Olson, C.T. Wang and N. Kulevsky, JOC, 32, 3227, (1967).
2.a. N.H. Potter, Diss. Abstr., B27, 3865, (1967).
2.b. M. Okawara, H. Sato, and E. Imoto, J. Chem. Soc. Japan. (Ind. Chem. Sec.), 58, 924, (1955). Chem. Abs., 50, 12878, (1956).
2.c. J.A. Cooper and W.A. Waters, J. Chem. Soc., B1967, (5) 455.
3. The Bu2O reaction was stirred for 12 hours at 3*C: It was then allowed to warm to 20*C and stirring was continued at that temperature for eight hours.
4. Values for yields are based on the number of moles of trichloroisocyanuric acid (the ether and water were always used in excess.)
5. In this reaction, an 18-fold molar excess of water was used.
6. This value is based on the conversion of benzyl ethyl ether to benzaldehyde and ethyl alcohol.
Act quickly or not at all.
(Active Asperger Archivist)
07-02-03 11:28
No 444048
(Rated as: excellent)
The Oxidation of Alcohols and Ethers Using Calcium Hypochlorite
Tetrahedron Letters, Vol. 23, No. 1, pp. 35-38, (1982)
Stephen O. Nwaukwa and Philip M. Keehn
Abstract:
Calcium hypochlorite, a relatively stable, and easily stored and used solid hypochlorite oxidant, was found to oxidize secondary alcohols to ketones in excellent yields. Primary alcohols gave esters where both the acid and the alcohol portions of the ester were derived from the alcohol. Ethers were oxidized to esters though only in moderate yields.
While carrying out studies on the Grob-type cleavage of gamma-hydroxysulphones2,3 we attempted the preparation of the corresponding hypochlorite by the action of sodium hypochlorite on 2-tosylmethyl cyclohexanol4 Instead of the hypochlorite, an excellent yield of the corresponding ketone (2-tosylmethylcyclohexanone) was obtained. Subsequent to this conversion, we independently found that this method was general for converting secondary alcohols to ketones by the use of sodium hypochlorite or commercially available Chlorox solutions. Though our conditions differed somewhat, the results were essentially the same as those reported by Stevens5.
The instability of sodium hypochlorite solutions, however, led us to consider other hypochlorite reagents which would be more stable and easier to handle. Calcium hypochlorite6 is a commercially available solid and is inexpensive. Since this reagent does not decompose significantly when stored without light in a desiccator7, carrying out oxidations by weighing the required amount of solid oxidant represented a more convenient method than using solutions which would frequently have to be titrated. We now wish to report our results concerning the use of Calcium hypochlorite as an oxidant.
Oxidations of secondary alcohols with this reagent proceed smoothly, and in excellent yields at 0*C in a solvent containing acetic acid. Our results are given in Table 1 for twelve compounds along with our initial results using sodium hypochlorite.8
A general procedure is outlined for the oxidation of l-menthol to l-menthone:
Thus, Menthol (3g, 19mmol) was dissolved in acetonitrile: acetic acid (3:2, 25ml) and added dropwise over a period of 10 minutes to a cooled (0*C) and stirred solution of calcium hypochlorite (1.84g, 12.7mmol) in water (40ml). Stirring was continued for 1 hr after which water (40ml) was added. The solution was extracted with DCM (4x30ml) and the organic layers washed with 10% sodium bicarbonate followed by an aqueous wash. After drying with Magnesium sulfate and evaporation of solvent, the crude product was distilled affording menthone (2.89g, 98%). The spectra (IR and NMR) were identical with those of authentic material.9,10
Oxidation of primary alcohols under identical conditions gave an aldehyde only in the case of benzyl alcohol11. Other primary alcohols gave esters as in Table 2. This table also includes our results on the oxidation of ethers to esters. Though the yields were not nearly as good for the alcohols, the data is reported because of the unusual and potentially useful transformation12. The ethers were oxidized under similar conditions as the alcohols except that the reactions were carried out at RT for 4-16 hours. Heating does not seem to increase yield.
We are presently carrying out studies to improve the yields on the ether to ester transformation and to utilize the primary alcohol oxidation for the preparation of lactones from alpha-w diols.
Table 1: The Oxidation of Secondary Alcohols Using Calcium and Sodium Hypochlorite.
| Run | Substrate | Product | %Yielda w/ Ca hypo | %Yield w/Na hypo | Reference |
| 1 | l-Menthol | l-Menthone | 98 | 98 | 9a,b |
| 2 | Borneol | Camphor | 98 | 99 | 9c |
| 3 | Norborneol | Norcamphor | 92 | --- | 10 |
| 4 | Cyclohexanol | Cyclohexanone | 91 | 98 | 9c |
| 5 | 2-tosylmethylcyclohexanol4 | 2-tosylmethylcyclohexanonec | 98 | 98 | --- |
| 6 | 3,5-dimethylcyclohexanol | 3,5-dimethylcyclohexanone | 93 | --- | 10 |
| 7 | 5-cholesten-3-ol | 4-cholesten-3-one | 91 | 91 | 10b |
| 8 | 3-pentanol | 3-pentanone | 97 | --- | 9d |
| 9 | 3-pentanol | 3-pentanone | --- | 87 | 9d |
| 10 | 2-octanol | 2-octanone | 80 | 99 | 9d |
| 11 | diphenylcarbinol | benzolphenone | 98 | --- | 9d |
| 12 | 2-tosylmethyl-1-phenylethanol4 | alpha-tosylmethylacetophenoned | --- | 98 | --- |
a. Isolated yield
b. DCM was used as solvent instead of acetonitrile for solubility reasons
c. IR, DCM (cm-1), 1710, 1320, 1150; NMR, CDCl3 (delta), 7.1-7.9, (m, 4H), 3.8-4.02 (dd, 2H) 2.5 (s, 3H), 1.5-2.1 (m, 9H); ms, m/e 266, 111.
d. MP: 130-131*C; IR, DCM (cm-1) 1690, 1315, 1150; NMR, CDCl3, (delta) 7.3-8.0 (m, 9H), 3.52 (s, 4H), 2.47 (s, 3H); ms, m/e 288.
Table 2: Oxidation of Primary Alcohols and Ethers Using Calcium and Sodium Hypochlorite.
| Run | Substrate | Product | %Yielda w/ Ca hypo | %Yield w/Na hypo | Reference |
| 1 | Benzyl alcohol | benzaldehyde | 98 | 98 | 10 |
| 2 | 1-pentanol | pentyl pentanoate | 83 | 91 | 10 |
| 3 | 1-hexanol | hexyl hexanoate | 98 | 98 | 9e |
| 4 | 3-methylbutanol | 3-methylbutyl isovalerate | 76 | 87 | 10 |
| 5 | ethyl alcohol | ethyl acetate | --- | b | 9b, 10 |
| 6 | ethyl ether | ethyl acetate | b | --- | --- |
| 7 | butyl ether | butyl butanoate | 40 | --- | 10 |
| 8 | THF | gamma-butyrolactone | 68 | --- | 9b, 10 |
| 9 | Tetrahydropyran | Delta-valerolactone | 56c | --- | 9b, 10 |
a. Isolated Yield
b Yield not calculated due to the volatility of the products but significant conversion was indicated by IR and NMR analysis.
c. Yield obtained by GC analysis
References:
1.---
2. M.F. Semmelhack and J.C. Tomesch, JOC, 42, 2657, (1977)
3. C.A. Grob and P.W. Schiess, Agnew. Chem. Int. Ed. Engl., 6, 1, (1967)
4. The synthesis of this alcohol will be described in a forthcoming publication. The correct elemental analysis has been obtained.
5. R.V. Stevens, K.T. Chapman and N.H. Weller, JOC, 45, 2030, (1980)
6. Obtained from Fisher Scientific Company, Typical analysis 67% Ca(OCl)2
7. Titrations were carried out over a period of two and one half months and there was no change in the concentration of the oxidant. Titrations were carried out as described in “A Textbook of Quantitative Inorganic Analysis”, 3rd ed. By Arthur I. Vogel. Published by J. Wiley and Sons, New York, N.Y. (1961)
8. Both Chlorox (5.25% oxidant) and freshly prepared NaOCl were used without significant difference. NaOCl was prepared by bubbling chlorine into a solution of NaOH as described by V. Boido and O.E. Edwards , Can. J. Chem. , 49, 2664, (1971)
9. a. C.J. Pouchert, The Aldrich Library of Infrared Spectra, Second Edition, published by Aldrich Chemical Co., Inc, Milwaukee, Wisconsin, (1975)
9. b. C.J. Pouchert, and John R. Campbell, The Aldrich Library of NMR Spectra Vol. II, published by Aldrich Chemical Co., Inc., Milwaukee, Wisconsin., (1974)
9. c. Merck Index, 8th ed., Merck and Co. Inc., N.J. (1968)
9. d. ibid, 9th ed. Merck and Co., Inc, N.J. (1976)
9. e. CA, 37, 5021
10. Dictionary of Organic Compounds, 5th ed. Oxford University Press, N.Y. (1969).
11. C.Y. Meyers, JOC, 26, 1046, (1961)
12. a. L.M. Berkowitz and P.N. Rylander, JACS, 80, 6682, (1958)
12. b. Yoshiko Kamiya and S. Takemuro, Chem. Pharm. Bull. 21, 1401, (1973)
13. N. C. Demo and N. H. Potter, JACS, 89, 3550, (1967)
Act quickly or not at all.
(Chief Bee)
07-03-03 19:19
No 444358
Production of
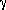 -butyrolactone by liquid phase oxidation of 1,4-butanediol
-butyrolactone by liquid phase oxidation of 1,4-butanediolMukhopadhyay S., Chandalia S.B.,
Indian J. Chem. Technol., Vol. 6, No. 4, 237–239 (1999) (../rhodium/djvu /miukhopadhy
Abstract
An alternative manufacturing process-scheme was developed to synthesize gamma-butyrolactone with very high selectivity. Ruthenium trichloride was used as a redox catalyst and sodium hypochlorite as the oxidizing agent. Different process parameters such as temperature, effect of pH, mode of reaction and catalyst loading were studied to develop the most suitable process conditions. Under best suitable conditions, at 83% conversion level of 1,4-butanediol, 97% selectivity to the desired lactone was achieved in 5 h.
This reference was kindly retrieved by lugh, now can somebody please type and post it in this thread? A DjVu plugin can be downloaded from http://www.lizardtech.com/download if you don't have one already.
(Hive Bee)
07-04-03 11:14
No 444525
(Rated as: excellent)
Indian Journal of Chemical Technology
Vol. 6, July 1999, pp. 237-239
S Mukhopadhyay & S B Chandalia
Dep. of Chemical Technology, Univ. of Mumbai, Matunga, Mumbai 400 019, India
recieved 25 May 1998; accepted 18 November 1998
An alternative manufacturing process-scheme was developed to synthesize gamma-butyrolactone with very high selectivity. Ruthenium trichloride was used as a redox catalyst and sodium hypochlorite as the oxidizing agent. Different process parameters such as temperature, effect of pH, mode of reaction and catalyst loading were studied to develop the most suitable process conditions. Under the best suitable conditions, at 83% conversion level of 1,4-butanediol, 97% selectivity to the desired lactone was achieved in 5 h.
Gamma-butyrolactone is used as an intermediate in the manufacturing of pyrrolidone derivatives, as a solvent for polymers, agro-chemicals, sun lotions and in printing inks. It is also used as an extractant in petroleum industries, as a stabiliser for chloro hydrocarbon and phosphorous-based pesticides and as a nematocide.
This is synthesized1-6 by vapour as well as liquid phase oxidation of 1,4-butanediol. In this work, an alternative method of production of gamma-butyrolactone starting from 1,4-butanediol was studied. Sodium hypochlorite was used as an oxidizing agent as it is cheap and could be produced in situ and ruthenium trichloride as the redox catalyst which could be recycled back.
Experimental procedure
Material
1,4-Butanediol, L. R. grade;
sodium hypochlorite, Technical grade;
ruthenium trichloride, Technical grade were used
Experimental setup
The experiments were carried out in a 250mL capacity borosilicate glass reactor provided with a stirrer, baffles, thermometer pocket and an addition funnel. The reactor was kept in a constant temperature ice water-bath.
Procedure
Predetermined quantity of 1,4-butanediol and catalyst were taken in the reactor and sodium hypochlorite was added dropwise from the addition funnel over a specified period of time at a particular temperature. 10% solution of NaOH was added simultaneously to maintain the pH at 8.2-8.9.
Analysis
Samples of reaction mixture were analysed by GC using a TCD
Conditions-Column 2 x 4m. OV-17 column; H2 flow rate : 12 mL/min (Carrier gas); TCD Block temperature : 156°C; Program : 60°C, 8°C/min ramp, 210°C, 15°C/min, 250°C, 5 min.
Estimation of aldehyde by oximation
The aldehyde was estimated by oximation using hydroxylamine hydrochloride. The hydroxyl amine hydrochloride solution was prepared in 95% ethanol and was adjusted to pH 3. Most of the aldehyde reacted with the reagent producing equivalent amount of hydrochloric acid.
The strongly alcoholic environment serves as a solvent for the carbonyl compound and its oxime. A sample of the reaction was taken in a 250 mL beaker, diluted with alcohol and dil HCl was added to adjust the pH of the solution to 3. Hydroxylamine hydrochloride solution was added and the reaction mixture was kept at 45°C for an hour. The liberated hydrochloric acid was estimated by titrating potentiometrically against standard NaOH solution. The amount of aldehyde present in the sample was calculated as,
g of aldehyde = (S x N x M)/1000
S = mL of standard NaOH required for titration.
N = Normality of NaOH solution.
M = Molecular weight of aldehyde.
isolation
After the stipulated reaction period the excess alkali was neutralized with dil HCl and water was removed by atmospherical distillation. Then the residual solution was fractionated under vacuum using a 4 ft column to obtain gamma-butyrolactone with 99.8% purity.
Results and Discussion
In this reaction pH is the most important parameter. At pH 8.2-8.9 sodium hypochlorite remains as OCl-, which is the true oxidizing agent at that pH. The reaction was studied in batch as well as semi-batch manner. When the reaction was done in a batch-mode then the conversion was only 65% whereas in semi-batch mode the conversion was 83% under identical conditions (Table 1). This was because of change in pH in batch-mode reaction. From Fig. 1 (*), the change of pH can be understood. To tackle this problem, it was therefore thought desirable to perform this reaction in a semi-batch manner by adding sodium hypochlorite and sodium hydroxide solution dropwise to the reaction mixture.
Table 1 - Batch mode vs. semi-batch mode
| Mode of reaction | % Conversion of 1,4-butanediol | % selectivity to gamma-butyrolactone |
| Batch | 65 | 97.3 |
| Semi-batch | 83 | 97.0 |
Reaction conditions: 1,4-butanediol, 0.316 gmol/L; sodium hypochlorite, 1.56 gmol/L; ruthenium trichloride, 0.0014 gmol/L; temerature, 30°C; reaction time 5 h.
To study the effect of temperature on the specific rate of reaction, the reaction was studied at different temperatures. It was observed that when the temperature was increased from 10-30°C (Fig. 2(*)) the conversion of 1,4-butanediol increased from 38 to 83%, the selectivity also increased from 60 to 97.3%, because at a lower temperature aldehyde formation was more. Even at room temperature there is always a chance of dissociation of sodium hypochlorite, so 100% utilisation of sodium hypochlorite was never achieved. At a temperature higher than 30°C, there is always a chance of a faster dissociation rate of sodium hypochlorite. At 35°C there was a slight decrease in conversion level though the selectivity remained the same and at 40°C the conversion was only 72% (Table 2). Thus a temperature of 30°C was proved to be most suitable to obtain the maximum conversion and selectivity.
The reaction was studied at different catalyst loading. When the catalyst loading was increased from 0.1% w/v to 0.3% w/v (fig. 3(*)) the conversion increased from 40% to 80% but the selectivity was almost the same.
Table 2 - Effect of temperature on the conversion and selectivity
| Temperature, °C | % Conversion of 1,4-butanediol | % selectivity to gamma-butyrolactone |
| 10 | 38 | 60.0 |
| 20 | 62 | 83.0 |
| 30 | 83 | 97.3 |
| 35 | 78 | 97.2 |
| 40 | 72 | 96.0 |
Reaction conditions: 1,4-butanediol, 0.316 gmol/L; sodium hypochlorite, 1.56 gmol/L; ruthenium trichloride, 0.0014 gmol/L; reaction time 5 h.
Comparision between this process vs existing processes
All the reported processes are mainly on the vapor phase oxidation of 1,4-butanediol. The operation hazard and installation cost of which are large. In vapour phase oxidation, there is always a chance of getting a range of byproducts; the separation of the desired product from the byproducts is again a problem and a major cost center.
In this liquid phase process-scheme, process conditions are manipulated in a way to get maximum selectivity to the desired product thus lowering the formation of byproducts.
Unlike vapour phase oxidation, the rate of oxidation in this process-scheme was high as the true oxidation agent was ruthenium tetroxide which formed in situ in the reaction mixture by reaction of ruthenium trichloride with water. As the ruthenium metal in catalytic amount (0.3% w/v) is being used, so the operation hazard is not severe. The cost of ruthenium is not a problem as ruthenium metal could be recycled by reacting the oxides with hydrochloric acid followed by hydrogenation. Sodium hypochlorite should be prepared in situ during the reaction by passing chlorine gas through sodium hydroxide solution thus it could lower the raw material costs. The reaction is eco-friendly as the concentration of the reactants were kept at very low concentration to minimize the operation hazards.
Conclusion
It was possible to produce butyrolactone with high selectivity. Under the best suitable conditions (temperature 30°C, time 5 h, reactant 0.3 gmol/L, NaOCl 1.56 gmol/L, catalyst loading 0.3% w/v) a conversion as high as 83% and a selectivity of 97% were achieved. An analytical method using TCD in gas chromatography was developed.
Acknowledgements
One of the authors (SM) is grateful to the University Grants Commission, New Delhi, for the award of a senior research fellowship.
References
1 Koyama H, Daicel chemical Industries ltd., Jpn Pat 02,255,668, (1990), cf. Chem Abstr, 114:122039.
2 Wolfe S, Hasan S K & Campbell J R, JCS Chem Commun, D (1970) 1420.
3 Plotkin J S, Patent US347647 (?), (1991), cf. Chem Abstr, 114:228733.
4 Murahashi S I, Naota T, Ito K, Maeda Y & Taki H, J Org Chem, 52 (1987) 4319.
5 Murahashi S I, Naota T, Ito K, Maeda Y, Tetrahedron Lett, 22 (1981) 5327.
6 Bekowitz L M & Rylander P N, J Am Chem Soc, 80 (1958) 6682.
(*) : For these figures you are referred to the above Djvu document, as I was unable to import these in this post.
Post 442416 (Rhodium: "That has been taken care of", Novel Discourse) : I prefer asian girls
http://www.poedecoder.com/Qrisse/works/d
(Active Asperger Archivist)
07-11-03 13:50
No 446457
(Rated as: excellent)
Oxidation Studies of Symmetrical and Unsymmetrial Ethers
Tetrahedron Vol. 27, pp 2671-2674, (1971)
E.C. Jeunge, M.D. Corey and D.A. Beal
Comparison of Trichloroisocyanuric Acid and Hypochlorous Acid Reagent
Abstract:
The study of the oxidation of symmetrical and unsymmetrical ethers in the direct conversions of ethers to esters by trichloroisocyanuric acid is reported. Initial work indicates that there is a selectivity in the oxidation of unsymmetrical ethers and that Newman’s “rule of six” may be an important factor in this selectivity. Hypochlorous acid which is a simpler positive halogenating agent and which could actually be the functioning oxidizing agent generated by hydrolysis of trichloroisocyanuric acid is not effective as a general oxidizing agent or substitute for trichloroisocyanuric acid. Although it does oxidize diethyl ether and THF.
The first simple convenient laboratory method for conversion of ethers to esters by the use of the inexpensive reagent, aqueous trichloroisocyanuric acid (TCIA), has been reported in a previous paper.1 Good yields are obtained with quantitative precipitation of insoluble and easily removable cyanuric acid. Various modifications of procedure may be employed.
With various reagents oxidation occurs fairly readily in the case of certain ethers such as THF2 and benzyl ethers;3 although in the latter case, cleavage of the ether linkage may occur.4 Thus, THF has been converted to gamma-butyrolactone by aqueous bromine4 or air oxidation.2 TCIA has been found to give similar results for conversion of THF to gamma-butyrolactone5 and cleavage of benzyl ethers to benzaldehyde.1 t-Butyl benzyl ether has been converted to t-butyl benzoate through ozonization by Erickson et al.6
Attempted photooxidation by Stenburg et al.7 of diethyl ether to ethyl acetate in the liquid phase by oxygen has given only a 3% yield of ester. Moreover, ozone has failed to yield ethyl acetate by reaction with diethyl ether. This ozonization has been examined to exclude it as a possible explanation for ester formation in the above reaction with oxygen.7 The rather expensive ruthenium tetroxide, however, has been found to serve successfully for conversion of ethers to esters, although its selectivity in the oxidation of unsymmetrical ethers was not investigated.8
Symmetrical ethers are converted to a single ester product. Initial study of unsymmetrical ethers presented here indicates that the product is selectivity determined by steric effect, namely, Newman’s “rule of six”. Thus, ethyl isobutyl ether yielded ethyl isobutyrate while ethyl isopropyl ether yielded isopropyl acetate.
At first sight, it would appear that oxidation would occur at the opposite organic groups because of stabilization of likely intermediates (free radical or carbonium ion) through hyperconjugation afforded by methyl substituents. The resulting product from ethyl isobutyl ether may be explained by Newman’s steric hinderance, that is, whiplike hindrance at the methylene part of the ethyl group by the terminal hydrogens of the methyls in the isobutyl group. The product from ethyl isopropyl ether may be explained by the lack of Newman’s steric hindrance and by the fact that the TCIA molecule is a fairly large one and subject to steric hindrance at the larger isopropyl group. Thus, the acetate was obtained instead of acetone which would be expected on oxidation of the isopropyl group. Oxidation of diisopropyl ether did yield acetone on oxidation by TCIA in the presence of water.
| TOa | Add | Temp (*C) | Time for addition (min) | Product | Yield (%) |
| Et2O and H2O | TCIA | 0-10 | 45 | Et Acetate | 49 |
| TCIA and H2O | Bu2O | 0-10 | 60 | Bu Butyrate | 50 |
| i-BuOEt and H2O | TCIA | 0-10 | 45 | Et i-butyrate | 83 |
| i-PrOEt and H2O | TCIA | 0-10 | 45 | i-Pr acetate | 55 |
| (i-Pr)2O and H2 | TCIA | 0-10 | 60 | acetone | 39 |
| OOEt and H2O | TCIA | 0-10 | 45 | OCHO | 53 |
| Et2O and H2O | Cl2 | 0-10 | 60 | Et acetate | 29c |
| THFb and H2O | Cl2 | 0-10 | 15 | gamma-butyrolactone | 5c |
| ” | Cl2 | 40 | 120 | ” | ” |
a TCIA is added slowly with intial reaction at 0-10*C. The possibility of reversing the addition is illustrated by the Bu2O oxidation.
b THF also reacts with TCIA and H2O to yield ester.1 caution—Adding THF direcly to excess TCIA once2 in an explosion.
c Amount of Cl2 consumed was not measured and no attempt was made to optimize yields.
Since it is known that upon hydrolysis, TCIA yields hypochlorous acid,9 attempts were made to oxidize several ethers with hypochlorous acid (chlorine-water). Limited investigation indicates that hypochlorous acid is not effective as a general substitute for TCIA. Chlorine-water was effective for conversion of diethyl ether and THF to ethyl acetate and butyrolactone, respectively. An ether with a longer alkyl chain was studied in order to check the generality of the chlorine-water oxidation. Dibutyl ether was more difficult to oxidize. Reaction of this ether with TCIA gave about 50% yield of ester and the reaction with hypochlorous acid failed. Repeated attempts to convert dibutyl ether to butyl butyrate by hypochlorous acid oxidation were unsuccessful. Butyl butyrate was not obtainable by distillation of, or evident from , gas chromatographic analysis of reaction products. Evidence of polymerization often appeared during distillation and traces of butyraldehyde were found in some of the reaction products. Even though attempted under a great variety of conditions, the reaction failed. Free radical conditions such as irradiation by ultraviolet light or catalysis by benzoyl peroxide at 60*C were fruitless and ferric chloride also failed to catalyze the reaction. Prohibiting the interference of HCl in the isolation of butyl butyrate by addition of t-butyl alcohol (as a scavenger) also failed to give the ester.
A sample taken of the mixture from the chlorine-water/diethyl ether run was subjected to a series of IR analyses. It was noted from these spectra that the CO peak (1710cm-1) increased with time as the OH peak (3100cm-1) decreased. At room temperature this sample evolved hydrogen chloride; however, no hydrogen chloride evolution occurred at 0*C. This data would suggest the presence of a temperature unstable intermediate such as a 1,1-halohydrin which could be stabilized as a H-bonded dimer in the low-molecule weight ether. Higher molecular weight ethers might yield, on the other hand, simple alpha-chloroethers which are known to dehydrohalogenate to give vinyl ethers and ultimately polymerize. Such formation of vinyl ethers is observed in the chlorination of ethers in the absence of water.10 These observations may account for the fact that hypochlorous acid does not serve as a general substitute for TCIA in direct conversion of ethers to esters.
Experimental:
Example 1:
Diethyl Ether and TCIA:
To anhydrous ether (35.5g) and distilled water (3.6g) stirred at 0-10*C was added over 45 minutes TCIA (7.06g). The mixture was stirred 2 hr at 0*C and then 10 minutes at RT. Cyanuric acid precipitated and was removed by filtration and the filtrate was shaken with saturated sodium bicarbonate. The ethereal layer was decanted and dried over magnesium sulfate. Distillation yielded EtOAc (1.89g, 49%) BP: 76; IR consistent with authentic ethyl acetate.
Example 2:
Dibutyl Ether and TCIA:
To water (50g) stirred at 0-10*C was added over 20 minutes TCIA (11.62g) followed by addition of dibutyl ether (100ml, 77g) over 1 hour at the same temperature. The mixture was then stirred at 10*C for 11hours and at 27*C for 8 hours. The cyanuric acid which precipitated was removed by filtration and the organic and aqueous layers were separated. The organic layer was shaken with saturated sodium bicarbonate and dried over anhydrous magnesium sulfate. Distillation of the organic layer yielded butyl butyrate (10.8g, 50.5%). BP: 78*C/23mmHg; The IR spectrum of the product was consistent with that found in Sadtler’s Index.
Example 3:
Ethyl Isobutyl Ether and TCIA:
The procedure was the same as Example 2. Ethyl isobutyl ether (15.3g), TCIA (34.6g), and water (9g) were used. Ethyl isobutyrate (14.4g, 83%) BP: 23*C/20mmHg; was obtained with IR identical to that in Sadtler’s.
Example 4:
Ethyl Isopropyl Ether and TCIA:
Again, procedure was as in Example 2. Ethyl isopropyl ether (72g), TCIA (19.6g), and water (4g) were used. Isopropyl acetate (55%), BP: 23*C/20mmHg; IR consistent with authentic sample.
Example 5:
Diisopropyl Ether and TCIA:
Diisopropyl ether (21.43g, 0.223mol) and water (100g, 5.55mol) were chilled to 0-10*C and TCIA (56.4g, 0.244mol) was added following the procedure used for diethyl ether. The mixture was stirred at 0-10*C for 6 hours and at RT for 3.5 hours. The cyanuric acid was filtered off and the filtrate neutralized with sodium bicarbonate. Distillation yielded acetone (6.65g, 39.4%); BP: 56.1*C; no isopropanol. IR confirmed with Sadtler’s.
Example 6:
Diethyl Ether and Chlorine-Water:
An excess of diethyl ether (106.5g) and distilled water (5g) were stirred rapidly at 0-10*C and chlorine was passed over the mixture for 1 hour followed by additional stirring for 3 hours. The mixture separated into an upper layer of 30ml and a lower layer of 83ml. The upper layer was neutralized with solid potassium carbonate and dried over calcium sulfate. Examination of this layer by IR indicated increase of the C=O peak at the expense of the O-H peak and the C-Cl peak with time. After the removal of the solvent by distillation, a residue of ethyl acetate (3.6g) was obtained. The lower layer was extracted several times with small quantities of water and dried over calcium sulfate. The extracts were combined, neutralized with solid potassium carbonate and extracted 3x20ml diethyl ether. The lower layer and etheric extracts were combined and distilled. Removal of the solvent by distillation left EtAc (3.6g).
Example 7:
THF and Chlorine-Water:
A slow stream of Cl2 gas was allowed to flow over a slowly stirred mixture of THF (55g, 077mol) and water (8g, 0.44mol) cooled to 0*C. After introducing Cl2 for 15 minutes, the temperature increased to 40*C. After 2 hours, chlorination was discontinued, and the mixture was allowed to stir overnight. The mixture separated into 2 layers, 15 ml of upper aq. Layer and 60ml of ethereal layer. The combined layers were mixed with 60ml of diethyl ether and extracted 4x with 5% sodium bicarbonate. The ether layer was discarded and the water layer was neutralized to a pH of approximately 5 with solid sodium bicarbonate. Precipitated NaCl was removed by filtration. Benzene (20ml) was added to the filtrate and water, HCl and THF were removed by azeotropic distillation with benzene for 48 hours. The benzene was then removed by distillation leaving a slurry of NaCl. The slurry was extracted several times with diethyl ether. The combined ether layers yielded after distillation, gamma-butyrolactone (3.16g, yield dependent on amount of chlorine used, not calculated); BP: 67*C/3mmHg; IR confimation positive.
Example 8:
Ethyl Benzyl Ether and TCIA:
The procedure is the same as in Example 1. The reactants were ethyl benzyl ether (57g, 0.42mol), water (6g, 0.33mol) and TCIA (23.5g, 0.10mol). The presence of chlorine was observed after 1/3 of the TCIA had been added. After addition, stirring was continued for 14 hours at 3*C. The cyanuric acid was removed by filtration. The ether layer was washed with water and neutralized with sodium bicarbonate solution. The ether layer was dried and a volatile fraction was removed through a 10cm vigreux column to give 15.3ml; BP: 75-77*C/12mmHg.
The residue from the distillation (1ml) was identified as ethyl benzoate using IR. A portion was converted to to the 2,4-dinitrophenylhydrazone of benzaldehyde, MP: 237-238* (2.86g, corresponds to 0.01mol benzaldehyde) The aldehyde was redistilled with a spinning band column to give the aldehyde (6.93g, 0.07mol); BP: 92-95*C/38mmHg; identified with IR; the combined yield represents (0.08mol) a 53.3% yield.
References:
1. aE.C. Juenge, M.D. Corey and D.A. Beal, Abstracts of Midwest Regional Meeting, Oct. 29, (1969); b E.C. Jeunge and D.A. Beal, Tetrahedron Letters, No. 55, 5819 (1968).
2. aM. Maincon and P. Chassaing (to Usines de Melle), US patent 2481765 (1949); Chem. Abstr., 44, 1532 (1950); bA. Robertson, Nature, London, 162, 153 (1948); Agnew. Chem., 61, 344, (1949); Chem. Abstr., 42, 8792, (1948); cUsines de Melle, British Patent 614392 (1948); Chem. Abstr., 44, 1531, (1948); also French Patent 990212 (1951); Chem. Abstr., 50, 7126, (1956).
3. R.L. Lovins, L.J. Andrews, and R.M. Keefer, JOC, 30, 4150, (1965); J.A. Cooper and W.A.Waters, J. Chem. Soc., B(5), 455 (1965)
4. N.C. Deno and N.H. Potter, JACS, 89, 3550, (1967)
5. E.C. Juenge, P.L. Spangler and W.P. Duncan, JOC, 31, 3836, (1966).
6. R.E. Erickson, R.T. Hansen, and J. Harkins, JACS, 90, 6777, (1968).
7. V.I. Stenburg, R.D. Olson, C.T. Wang and N. Kulevsky, JOC, 32, 3227, (1967).
8. L.M. Berkowitz and P.N. Rylander, JACS, 80, 6682, (1958).
9. A.P. Brady, K.M. Sancier, G. Sirine. Ibid., 85, 3101, (1963).
10. G. Sosnovsky, Free Radical Reactions in Preparative Org. Chem., Macmillan, N.Y., (1964)
Act quickly or not at all.
(Stranger)
07-12-03 16:55
No 446714
(Rated as: excellent)
4. Production
Dehydrogenation of 1,4-Butanediol [110-63-4] (® Butanediols, Butenediol, and Butynediol). The Reppe process for manufacturing butyrolactone involves the endothermic dehydrogenation of 1,4-butanediol in the gas phase. This process is used by BASF, ISP, and Lyondell.
Preheated 1,4-butanediol vapor is introduced into a hot stream of circulating hydrogen and passed at atmospheric pressure through a bed of copper catalyst at temperatures between 180 and 240 °C (Figure (1)). The yield of butyrolactone is approximately 95 %. The reaction takes place via g-hydroxybutyraldehyde [25714-71-0] [7].
The byproduct hydrogen off-gas requires only simple purification before reuse (e.g., catalytic methanization of carbon monoxide impurities). The crude butyrolactone separated from the recycle gas stream contains small amounts of byproducts, including 1,4-butanediol, butyric acid, and high boilers, from which butyrolactone is separated by distillation.
Butyrolactone itself is noncorrosive and can be handled in carbon steel apparatus. However, where parts of the synthesis or distillation vessels and pipes come into contact with hot crude product containing butyric acid, they must be made of stainless steel.
Hydrogenation of Maleic Anhydride [108-31-6]. In the preparation of butyrolactone by hydrogenating maleic anhydride, molten maleic anhydride is fed into a preheated circulating stream of hydrogen and passed under a pressure of 6 – 12 MPa at 160 – 280 °C over a nickel-containing catalyst [8].
The reaction takes place via succinic anhydride [108-30-5] and can, by choice of the conditions, be continued to produce tetrahydrofuran [109-99-9]. The excess hydrogen is washed with water and recycled to the synthesis. Byproducts contained in the butyrolactone are separated out of the circulating gas: propanol [71-23-8], butanol [71-36-3], propionic acid [79-09-4], and butyric acid [107-92-6]. The butyrolactone is separated from these by distillation.
Because of the acids present, both the synthesis apparatus and the distillation apparatus must be made of stainless steel. The Japanese manufacturer Mitsubishi Chemical Corporation [9] uses this process.
Hydrogenation of Maleic Esters. New processes for the production of 1,4-butanediol and tetrahydrofuran starting from maleic anhydride via dimethyl maleate have been developed in the past few years (® Tetrahydofuran). They offer the possibility of extracting butyrolactone, which is an intermediate in these processes.
In a process developed by Kvaerner Process Technology (KPT, London) [10] dimethyl maleate [624-48-6] is produced in a first step from maleic anhydride and methanol with a strongly acidic ion exchanger as catalyst. The resulting dimethyl maleate is hydrogenated in the gas phase on a Cu-containing catalyst at a pressure of 2 – 8 MPa at 150 – 250 °C and gives a mixture of 1,4-butanediol, tetrahydrofuran, butyrolactone, and a small amount of the intermediate dimethyl succinate [106-65-0].
Butyrolactone and dimethyl succinate can be recovered as an azeotrope and recycled to the hydrogenation stage to obtain complete conversion to 1,4-butanediol and tetrahydrofuran. Alternatively the azeotrope can be refined by distillation to recover pure butyrolactone. The amount of butyrolactone depends on the pressure and temperature in the hydrogenation step, which influence the equilibrium between 1,4-butanediol and butyrolactone. Under the conditions described above it may vary from 5 to 50 %.
The new process has been licensed by KPT several times. The first commercial plants using this process are expected to come on stream in 2000.
A proprietary process practised by Eurodiol, a Belgian company of the SISAS group also starts from dimethyl maleate, which is hydrogenated in the gas phase at 1 – 2 MPa to give a mixture of butyrolactone and tetrahydrofuran in variable proportions. Butyrolactone and tetrahydrofuran are recovered as pure products by distillation, while the byproduct azeotrope butyrolactone/dimethyl succinate can be recycled for full conversion to butyrolactone and tetrahydrofuran or hydrogenated in a subsequent hydrogenation step in the liquid phase to give 1,4-butanediol and additional tetrahydrofuran.
Other Processes. Processes via tetrahydrofuran [11], dihydrofuran [12], acetylene [13], [14], butynediol [15], olefins [16][17][18], butadiene [8], or by carbonylation [19][20][21] are not industrially important.
Producers.
Butyrolactone is manufactured by BASF (Ludwigshafen, Germany and Geismar, USA), ISP (Calvert City and Texas City, USA), Lyondell (Channelview, USA), MCC (Mizushima, Japan) and Eurodiol (Feluy, Belgium).
[7] S. Oka, Bull. Chem. Soc. Jpn. 35 (1962) 986 – 989.
[8] Mitsubishi Petrochemical, DE-OS 1593073, 1966; DE-OS 1901870, 1969 (T. Asano, J. Kanetaka).
[9] J. Kanetaka, T. Asano, S. Masumune, Ind. Eng. Chem. 62 (1970) 24 – 32. T. Yoshimura, Chem. Eng. N.Y. 76 (11. Aug. 1969) 70 – 72; Chem. Week 104 (1969) 63 – 72. S. Minoda, M. Miyajima, Hydrocarbon Process. 49 (1970) no. 11, 176 – 178.
[10] M. W. M. Tuck, M. A. Wood, C. Rathmell, P. H. E. Eastland: "Butane to Butanediol: The emergence of a new Process Route," AIChE 1994 Spring International Meeting.
[11] Quaker Oats, US 3074964, 1961 (A. P. Dunlop, E. Sherman).H. Hara, JP-Kokai 7887347, 1978.
[12] BASF, DE 4 339 269, 1993 (R. Pinkos, R. Fischer).
[13] BASF, WO 9 707 111, 1995 (M. Heider et al.).
[14] BASF, DE 19 530 549, 1995 (M. Heider, T. Ruehl, J. Henkelmann, S. Stutz).
[15] Y. Shvo, Y. Blum, J. Organomet. Chem. 238 (1982) C 79 – C 81.
[16] Toa Nenryo Kogyo K.K., JP-Kokai 75 154 237, 1975 (Y. Okumura, Y. Nagashima).
[17] Nat. Dist. and Chem. Corp., US 4 247 467, 1978 (J. H. Murib).
[18] Standard Oil, US 4 238 357, 1980 (Th. Haase, F. A. Pesa).
[19] Texaco, US 3 061 614, 1958 (W. M. Sweeney, J. A. Patterson).
[20] The British Petroleum Company, EP 176 370, 1984 (H. Alper, D. J. H. Smith).
[21] ARCO Chem. Technology LP, US 5 401 857, 1994 (D. Armstead, R. A. Grey).
Taken from Ullman's Encyclopedia of industrial chemistry
(Chief Bee)
10-23-03 11:37
No 466341
(Rated as: excellent)
A Novel Production of
 -Butyrolactone Catalyzed by Ruthenium Complexes
-Butyrolactone Catalyzed by Ruthenium ComplexesY. Hara, H. Kusaka, H. Inagaki, K. Takahashi, K. Wada
Journal of Catalysis, 194(2), 188-197 (2000) (../rhodium/pdf /gbl.succinic
DOI:10.1006/jcat.2000.2945
Abstract
 -Butyrolactone (hereafter abbreviated GBL) is produced by the two-stage hydrogenation of maleic anhydride (MAH) in the liquid phase: the hydrogenation of MAH to succinic anhydride (SAH) in the first stage and the subsequent hydrogenation of SAH to GBL in the second stage. The latter hydrogenation has been studied using a homogenous catalyst. A novel ruthenium catalyst system consisting of Ru(acac)3, trioctylphosphine, and p-toluenesulfonic acid (p-TsOH) was developed for hydrogenating the SAH, which exhibited excellent catalytic performance, exceeding 95% selectivity for GBL and higher activity than that reported in the literature. It was found that p-TsOH plays an important role not only in enhancing the reaction rate, but also in improving selectivity. p-TsOH induces a structual change in the Ru complexes, leading to the cationic change which shows higher catalyst activity. It also prevents the undesired side reaction catalyzed by free trioctylphosphine thus resulting in high selectivity for GBL. A process to produce GBL was investigated. Some novel features of this process include the external preparation method of the Ru complex, the coupling reaction, and the separation to remove H2O, a product of hydrogenation of SAH, to increase the reaction rate. A catalyst recovery system was also developed to recover over 90% of the catalyst.
-Butyrolactone (hereafter abbreviated GBL) is produced by the two-stage hydrogenation of maleic anhydride (MAH) in the liquid phase: the hydrogenation of MAH to succinic anhydride (SAH) in the first stage and the subsequent hydrogenation of SAH to GBL in the second stage. The latter hydrogenation has been studied using a homogenous catalyst. A novel ruthenium catalyst system consisting of Ru(acac)3, trioctylphosphine, and p-toluenesulfonic acid (p-TsOH) was developed for hydrogenating the SAH, which exhibited excellent catalytic performance, exceeding 95% selectivity for GBL and higher activity than that reported in the literature. It was found that p-TsOH plays an important role not only in enhancing the reaction rate, but also in improving selectivity. p-TsOH induces a structual change in the Ru complexes, leading to the cationic change which shows higher catalyst activity. It also prevents the undesired side reaction catalyzed by free trioctylphosphine thus resulting in high selectivity for GBL. A process to produce GBL was investigated. Some novel features of this process include the external preparation method of the Ru complex, the coupling reaction, and the separation to remove H2O, a product of hydrogenation of SAH, to increase the reaction rate. A catalyst recovery system was also developed to recover over 90% of the catalyst.Succinic Anhydride -> GBL + THF
Catalyst: Hydrous zirconium oxide (IPA, 280°C)
Subject Studied: Product distribution
Takahashi, Kyoko; Shibagaki, Makoto; Matsushita, Hajime; Bull.Chem.Soc.Jpn. 65(1), 262-266 (1992)
Succinic Anhydride -> GBL
Reagent: Lithium borohydride (THF, 25°C, 15 min, 68%)
Narasimhan, Srinivasan Heterocycles 18, 131-135 (1982)
Succinic Anhydride -> GBL
Reagent: Na/Hg, HCl(aq)
Fichter; Herbrand; Chem. Ber. 29, 1193 (1896)
(Chief Bee)
03-14-04 12:57
No 495126
(Rated as: excellent)
An aqueous silica gel disperse electrolysis system. N-Oxyl-mediated electrooxidation of alcohols
Hideo Tanaka, Yusuke Kawakami, Kentaro Goto and Manabu Kuroboshi
Tetrahedron Letters 42(3), 445-448 (2001) (../rhodium/pdf /bdo2gbl.elec
DOI:10.1016/S0040-4039(00)01979-1
Abstract
N-Oxyl-mediated electrooxidation of alcohols was performed in an aqueous silica gel disperse system. The newly devised electrolysis system offers an organic solvent-free and operationally simple procedure for oxidation of alcohols and could be successfully applied to kinetic resolution of sec-alcohol as well as enantioselective oxidation of meso-1,4-diol affording optically active gamma-lactone.
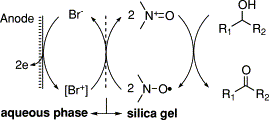
References
[1]
 (a) Semmelhack, M. F.; Chou, C. S.; Cortes, D. A. J. Am. Chem. Soc. 1983, 105, 4492.
(a) Semmelhack, M. F.; Chou, C. S.; Cortes, D. A. J. Am. Chem. Soc. 1983, 105, 4492.  (b) Bobbit, J. M.; Hung, Q. T.; Ma, Z. J. Org. Chem. 1993, 58, 4837.
(b) Bobbit, J. M.; Hung, Q. T.; Ma, Z. J. Org. Chem. 1993, 58, 4837.  (c) Yanagisawa, Y.; Kashiwagi, Y.; Kurashima, F.; Anzai, J.; Osa, T.; Bobbit, J. M. Chem. Lett. 1996, 1043.
(c) Yanagisawa, Y.; Kashiwagi, Y.; Kurashima, F.; Anzai, J.; Osa, T.; Bobbit, J. M. Chem. Lett. 1996, 1043.  (d) Kashiwagi, Y.; Yanagisawa, Y.; Kurashima, F.; Anzai, J.; Osa, T.; Bobbit, J. M. J. Chem. Soc., Chem. Commun. 1996, 2745.
(d) Kashiwagi, Y.; Yanagisawa, Y.; Kurashima, F.; Anzai, J.; Osa, T.; Bobbit, J. M. J. Chem. Soc., Chem. Commun. 1996, 2745.  (e) Kashiwagi, Y.; Yanagisawa, Y.; Kurashima, F.; Anzai, J; Osa, T. Tetrahedron Lett. 1999, 40, 6469.
(e) Kashiwagi, Y.; Yanagisawa, Y.; Kurashima, F.; Anzai, J; Osa, T. Tetrahedron Lett. 1999, 40, 6469.[2]
 (a) Inokuchi, T.; Matsumoto, S.; Nishiyama, T.; Torii, S. J. Org. Chem. 55, 462 (1990) (../rhodium/pdf
/electrooxida
(a) Inokuchi, T.; Matsumoto, S.; Nishiyama, T.; Torii, S. J. Org. Chem. 55, 462 (1990) (../rhodium/pdf
/electrooxida (b) Torii, S.; Inokuchi, T.; Matsumoto, S.; Saeki, T.; Oki, T. Bull. Chem. Soc. Jpn. 1990, 63, 852.
(b) Torii, S.; Inokuchi, T.; Matsumoto, S.; Saeki, T.; Oki, T. Bull. Chem. Soc. Jpn. 1990, 63, 852.  (c) Inokuchi, T.; Matsumoto, S.; Torii, S. J. Org. Chem. 1991, 56, 2416 (1991) (../rhodium/pdf
/electrooxida
(c) Inokuchi, T.; Matsumoto, S.; Torii, S. J. Org. Chem. 1991, 56, 2416 (1991) (../rhodium/pdf
/electrooxida (d) Inokuchi, T.; Liu, P.; Torii, S. Chem. Lett. 1994, 1411.
(d) Inokuchi, T.; Liu, P.; Torii, S. Chem. Lett. 1994, 1411.  (e) Kuroboshi, M.; Yoshihisa, H.; Cortona, M. N.; Kawakami, Y.; Gao, Z.; Tanaka, H. Tetrahedron Lett. 2000, 41, 8131–8135.
(e) Kuroboshi, M.; Yoshihisa, H.; Cortona, M. N.; Kawakami, Y.; Gao, Z.; Tanaka, H. Tetrahedron Lett. 2000, 41, 8131–8135.[3] Involving HO(CH2)4CO2(CH2)4CO2H, HO2C(CH2)4CO2(CH2)5OH, etc.
[4] S.D. Rychnovsky, T.L. McLernon and H. Rajapakse. J. Org. Chem. 61, 1194 (1996) (../rhodium/pdf /electrooxida
[5] Determined by HPLC, column: CHIRALCEL-OD (4.6x50 mm), mobile phase: hexane/2-propanol (90/1), flow rate: 0.5 ml min-1.
[6] T. Ishikawa, Y. Oku and K.-I. Kotake. Tetrahedron 53, 14915–14928 (1997) (../rhodium/pdf /electrooxida
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
04-14-04 12:00
No 500826
(Rated as: excellent)
Convenient Synthesis of Lactones by the Reaction of Diols with N-Haloamides
Shuji Kondo, Shinya Kawasoe, Hideo Kunisada, and Yasuo Yuki
Synthetic Communications, 25(5), 719-724 (1995) (../rhodium/pdf /bd2gbl.haloa
Abstract
Reaction of 1,4-butanediol or 1,5-pentanediol with N-haloamides such as N-chlorosuccinimide, N-bromosuccinimide, N-bromoacetamide, isocyanuric chloride, and N,N-dichlorobenzenesulfonamide under mild conditions gave
 -butyrolactone or
-butyrolactone or 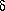 -valerolactone, respectively, in high yields.
-valerolactone, respectively, in high yields.____ ___ __ _
Preparation of N-Bromo-Acetamide from Acetamide and elemental Bromine
Organic Syntheses, CV 4, 104 (http://www.orgsyn.org/orgsyn/prep.asp?p
The Hive - Clandestine Chemists Without Borders
(acetaminophanatic)
04-15-04 20:28
No 501031
In the "Mechanism and Synthetic Utility of the Oxidative Cleavage of Ethers by Aqueous Bromine" paper (JACS 1967,3350), where they get a (claimed) quantitative yield of GBL from THF using a 4x excess of bromine, in the penultimate paragraph, they mention that chlorine quantitatively broke ethyl ether into acetic acid. Which is exactly what the bromine did.
It seems to ning that the secret to the oxidation of THF with halogen agents is:
1. Time...there is a competitive halogenation reaction at work, but it's slower than the oxidation. Also, the lactone formed by oxidation is deactivated, but it will still oxidize too. Figure out a good reaction time and as soon as the reaction finishes, quench it! (Above paper: One day)
2. Darkness. Free radicals --> baaad. Ionic attack --> good. Wrap your beaker with tin foil. We don't like halogenated lactones. The above paper did this, and they claim quantitative yields of lactone. Let's follow their example.
3. pH control. Bleach, chloramines, halogens and water have such complicated interactions. pH is an essential controlling factor in determining what species are at work in solution. The above paper uses acetate buffer. Chloramines like TCCA have the handy advantage of eating their own HX acid to produce more HOX. pH 5 is what they used for bromine. I think chlorine's curve is one or two pH lower than bromine, so that would bee about pH 4 for bleach or TCCA.
4. Water! It seems obvious, but that extra oxygen, where does it come from? Water! I think I just saw a paper where they reacted 50 g of THF with TCCA, but it only contained 6 g of water! And they got a low yield of GBL. What a surprise!
5. Water! Don't use an alcohol as a solvent unless you want to waste large quantities of oxidizer. If it reacts with an ether, you can be damn sure it will attack alcohols. Acetone will work as a cosolvent, as long as you don't mind a few percent chloroacetone in your workup. On the other hand, this could be to your advantage, as the acetone or MEK could perhaps sponge up the chlorine radicals that would otherwise attach themselves to your desired product. But THF is pretty soluble in water already, right?
So ning's suggested procedure, not knowing the real way of light, would be:
1 mole THF (72g) mixed with 50 ml water to eat peroxides
1.2 moles Ca(OCl)2 (172g)
Dissolve the Ca(OCl)2 into 800 ml water stirred at room temp in big-ass flask wrapped with aluminum foil. Acidify to pH 4-5 or so with acetic acid (or HCl?), then mount a dropping funnel on top with the THF and start dripping the THF, keeping the temperature down. When the addition's done, let it stir for a few hours until the flask is completely cool. Then quench the reaction with something (bisulfite?) and extract however you like. I guess I'd use steam distillation, since that'd probably not take anything but the good stuff over, and the dum water would have to be removed anyway. Dare I hope for 86 g of lactone? Probably a regular distillation would have to bee performed just in case those dirty chlorinated lactones make it over too.
Thoughts?
Catching a buzz @ the Hive
(Chief Bee)
07-06-04 16:30
No 517834
(Rated as: good read)
This article has been mentioned before in Post 53160 (psychokitty: "Re: THF -> GHB Research", Chemistry Discourse)
Selective oxidation of alcohols and oxidative lactonization of diols with trichloromelamine
Shuji Kondo, Mari Ohira, Shinya Kawasoe, Hideo Kunisada, Yasuo Yuki
J. Org. Chem. 58, 5003-5004 (1993) (../rhodium/pdf /bd2gbl.trich
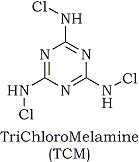
Trichloromelamine (TCM) can be viewed as a positive halogen compound, because the 1,3,5-triazine ring possess strong electron-withdrawing character. However, there are few papers concerning the utilization of TCM in organic synthesis. We report here a simple and selective oxidation of alcohols to the corresponding carbonyl compounds and oxidative lactonization of diols with TCM under mild conditions.
Table II
| Alcohol | Solvent | Time | Product | Yield |
| 1,4-BD | CH2Cl2 | 12h | GBL | 87% |
| 1,4-BD | CHCl3 | 12h | GBL | 74% |
| 1,4-BD | CH3CN | 12h | GBL | 68% |
| 1,4-BD | Pyridine | 12h | - | 0%* |
Oxidation of 1,4-butanediol (3) with TCM in methylene chloride afforded γ-butyrolactone (4), in 87% yield (Table II).
Experimental
Trichloromelamine (TCM) was commercially available and used as received. Starting alcohols were purified by distillation or recrystallization.
Oxidative Lactonization of l,4-Butanediol
To a mixture of 900 mg (10 mmol) of 1,4-butanediol and 60 mL of methylene chloride was added 4.69 g (20 mmol) of TCM. The mixture was stirred for 12 h at room temperature. After filtration,the solvent was removed under reduced pressure. The residue was purified by column chromatography (Wakogel C-200 eluent: methylene chloride) to give an oil (749 mg). The spectral data of the product agreed with those of an authentic sample of γ-butyrolactone.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
07-25-04 15:11
No 521602
(Rated as: good read)
Hi!
This is a dissertation about the catalytic gas-phase oxidation of maleic acid dimethyl esters on CuO-catalyst, and seems to be essentially the same like described a few posts earlier by OcoteaCymbarum (Post 446714 (OcoteaCymbarum: "Industrial production of gbl", Methods Discourse), but of course A BIT more verbose (127 pages
Here's a translation of part of the introduction:
"Gasphasenhydrierung von Maleinsaeuredimethylester zu 1,4-Butandiol, gamma-Butyrolacton und Tetrahydrofuran an Kupfer-Katalysatoren
(dissertation for the graduation to academical degree "Doctor of Science", by J.H. Schlander, University of Karlsruhe
{translation begin}
It is the goal of the present work to place a corner stone in systematic analysis of the multiple-stage gas phase hydration of maleic acid dimethyl esters on copper catalysts. To accomplish this, it was necessary to design and build a pilot plant which allowed for kinetic measurements inside a integral driven tube reactor.
With activated Cu/ZnO catalyst (selected based on orientating measurements) the product diversification is identified as function of the following parameters: pressure, temperature and reaction time.
Besides of maleic acid dimethyl esters, the intermediate products succinic acid dimethyl ester, GBL and 1,4-BDO were also used as starting compounds. The goal of the measurements is to help understanding the process of gas phase hydration of maleic acid dimethyl ester. Furthermore the measurements give information about how far the thermodynamic equilibrium between GBL and 1,4-BDO affects the ratio of these substances in the product stream.
Based on this data, it is shown that the system, based on a reaction matrix, can be described quantitatively with the help of simple basic kinetic approach.
Finally, a further development of the catalysts with regard to enhanced activity while maintaining same or better selectivity regarding value products is accomplished.{translation end}
Very interesting, but unfortunately the text is >120 pages - else I would've translated the whole lot..
I uploaded the PDF, for the interested amongst us....

Greetz A
Pleased to meet you hope you get my name.
But whats puzzlin you is the nature of my game!
(Hive Bee)
08-06-04 12:53
No 524030
../rhodium/pdf /alcohols2meth
I had the idea that the mentioned article could be useful when it comes to converting BDO to GHB - but I'm a bit unsure: the product from oxidizing 1,4-butanediol, 4-methyl-hydroxybutanoate, looks more like it could result in valerolactone - or is it already active by itself?
Is there a convenient way of removing the methyl? - Then this could well become a winner!!! (simple H2O hydrolysis maybe? ethylformate dissociates into EtOH and HCOOH upon prolonged contact with water, for example..)
BTW if double oxidation (at both OHs) was the main reaction to occur, then this would give dimethyl malonate from 1,3-propanediol, right? The starting compound being used in the article I wrote about in my previous post...
Greetz A
Pleased to meet you hope you get my name.
But whats puzzlin you is the nature of my game!
(Hive Bee)
08-07-04 05:14
No 524041
What do you mean by:
...the product from oxidizing 1,4-butanediol, 4-methyl-hydroxybutanoate, looks more like it could result in valerolactone...
You can't get any 4-methyl-hydroxybutanoate by the oxidation of 1,4-butanediol. You can only get methyl 4-hydroxy-butanoate by the reaction as you propose it. Though in my opinion you would get mostly just GBL. The methyl ester just like the lactone should hydrolyze with the aid of sodium hydroxide in sodium GHB salt.
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Chief Bee)
08-07-04 07:50
No 524057
Maybe this diagram will help you understand why there is a vast difference between the ester Methyl 4-Hydroxybutanoate (the product in the hypochlorite oxidation of butanediol in methanol) and the hydroxy-acid 4-Methyl-4-Hydroxybutanoate (the free acid corresponding to gamma-valerolactone).
gamma-Valerolactone and 4-Methyl-4-Hydroxybutanoate are active, see ../rhodium /4-methy
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
08-07-04 20:05
No 524199
sorry for my typo - meant methyl-4-hydroxybutanoate, not 4-methyl-4-OH-...
So 4-hydroxy-butanoic acid methyl ester hydrolyzes to 4-hydroxybutyric acid when treated with hot aequ. NaOH?
And upon treatment of 1,4-BDO with CaOCl/CH3OH, one would get a mixture of 4-hydroxybutanoic acid methyl ester and gamma-butyrolactone? Which is completely turned to GHB by the usual lye procedure?
Did I get that right so far?
Greetz A
Pleased to meet you hope you get my name.
But whats puzzlin you is the nature of my game!
(Chief Bee)
08-08-04 05:52
No 524240
Yes, everything you just said is correct. Just keep in mind that the basic hydrolysis goes like this:
4-hydroxy-butanoic acid methyl ester + NaOH -> Sodium 4-hydroxybutyrate + Methanol
You should remove that methanol before ingesting any of the resulting NaGHB.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
08-08-04 13:32
No 524296
Good old FSE revealed the following: Post 436497 (Rhodium: "NMP/GBL/dimethylmaleate separation", Chemicals & Equipment)
Same principle behind it, isn't it? The ester gets hydrolyzed and only the "GBL"-remainder of its molecule rearranges upon acidification, while CH3OH is formed?
Greetz, A
Pleased to meet you hope you get my name.
But whats puzzlin you is the nature of my game!
(Chief Bee)
08-08-04 18:53
No 524343
Yes, that's one way of expressing it - with the difference being that there is only one ester present in this case.
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
09-21-04 11:51
No 532536
(Rated as: excellent)
Oxidative Esterification of Primary Alcohols by NaBrO3/NaHSO3 Reagent in Aqueous Medium
Kiyoshi Takase, Haruyoshi Masada, Osamu Kai, Yutaka Nishiyama, Satoshi Sakaguchi, and Yasutaka Ishii
Chemistry Letters 871-872 (1995) (../rhodium/pdf /bd2gbl.nabro
Abstract
NaBrO3 combined with NaHSO3 was found to be an efficient reagent for the oxidative esterification of primary alcohols. Thus, a variety of esters was prepared from primary alcohols, aldehydes, and acetals in aqueous medium under mild conditions. Treatment of α,ω-diols with NaBrO3/NaHSO3 reagent afforded the corresponding lactones and/or dicarboxylic acids in fair yields.
____ ___ __ _
Selective, Heterogeneous Oxidation of Alcohols and Diols with Potassium Permanganate
Charles W. Jefford and Ying Wang
J. Chem. Soc. Chem. Commun., 634-635 (1988) (../rhodium/pdf /bd2gbl.kmno4
Abstract
Primary alcohols can be conveniently oxidized to carboxylic acids using solid KMnO4/CuSO4·5H2O/KOH in an organic solvent; 1,4- and 1,5-diols can be selectively oxidized to the corresponding lactones using appropriate mixtures of KMnO4/CuSO4·5H2O without added base.
____ ___ __ _
Oxidation of Alcohols with Peracetic Acid in Ethyl Acetate in the Presence of Sodium Bromide
Takashi Morimoto, Masao Hirano, Takayoshi Hamaguchi, Masahide Shimoyama, and Xiumin Zhuang
Bull. Chem. Soc. Jpn., 65, 703-706 (1992) (../rhodium/pdf /bd2gbl.perac
Abstract
Aliphatic primary alcohols and 1,ω-diols were oxidized to the dimeric esters and lactones, respectively, by peracetic acid in ethyl acetate in the presence of sodium bromide under mild conditions. Aliphatic secondary and benzylic alcohols were converted smoothly to the corresponding carbonyl compounds by the same system.
The Hive - Clandestine Chemists Without Borders