(Chief Bee)
02-26-03 04:19
No 411744
(Rated as: excellent)
According to Chem. Ber. 60, 1050-69 (1927) (../rhodium/djvu /hydratropal
I need help to translate the pertinent parts of the above article though (the mechanism discussion + experimental).
DjVu browser plugin (http://www.djvu.com/download/)
(Stoni's sexual toy)
02-26-03 17:01
No 411949
I remember seeing a patent where hydratropic aldehyde was rearranged in the vapor phase, by using solid catalysts. Maybe someone remembers the patent numbers? I know I wrote it down somewhere, but can't find it anymore.
I'm not fat just horizontally disproportionate.
http://www.whatreallyhappened.com
(Chief Bee)
02-26-03 22:21
No 412032
Osmium: You are probably looking for Patent US4694107. Can you please help with the translation of the above?
(Chief Bee)
03-02-03 06:33
No 412948
(Rated as: excellent)
Once upon a time, close to a hundred years ago, it seems like the frenchmen invented a simple way of turning Safrole to MDP2P as well as alpha-methylstyrene to P2P using something as simple as hypoiodous acid (HOI) and simple heating with acids & bases. However, to obscure their discovery as much as possible for the rest of the world, they encrypted their findings by publishing it in french (PGP was not available at the time). Luckily we have cryptography experts here at the Hive (Hypo & Chimimanie) who are specializing in this ancient cryptographic language, so we may soon find out what the frenchmen's secret was...
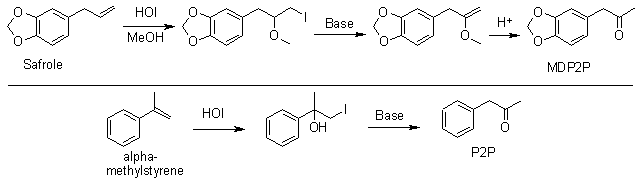
Bull. Soc. Chem. France 310-321 (1908) (../rhodium/djvu /tiffeneau52
Bull. Soc. Chem. France 322-331 (1908) (../rhodium/djvu /daufresne54
(Chief Bee)
07-12-03 19:13
No 446645
(Rated as: excellent)
Benzene Hydrocarbons with a Pseudo Allyl Side Chain: Methovinylbenzene and its Homologues. Study of Certain Molecular Migrations. Second Part. Study of Molecular Transpositions which Accompany the Transformation of
 -Glycols and their Derivatives into Aldehydes and Ketones.
-Glycols and their Derivatives into Aldehydes and Ketones.Tiffeneau, M.
Ann. chim. phys., [8], Vol 10, pp 322-78 (1908) (../rhodium/djvu /tiffeneau.c
Abstract
This paper consists of a continuation of the experimental study and theoretical consideration of certain migrations which take place in derivatives of glycols under the influence of various reagents. It was previously observed (Ann. chim. phys. [8], 10, 145-98) that halohydrins of
 -glycols, when treated with alkalies and then distilled, gave first an oxide and then an aldehyde, thus but with AgNO3, or HgO a more deep-seated transformation took place, resulting in the migration of a hydrocarbon radicle. Thus, from the iodohydrin of methylphenylglycol, phenylacetone was obtained according to PhCOH(Me)CH2I
-glycols, when treated with alkalies and then distilled, gave first an oxide and then an aldehyde, thus but with AgNO3, or HgO a more deep-seated transformation took place, resulting in the migration of a hydrocarbon radicle. Thus, from the iodohydrin of methylphenylglycol, phenylacetone was obtained according to PhCOH(Me)CH2I  PhCH2COMe. The author has studied successively the glycols, the oxides of ethylene and the halohydrins.
PhCH2COMe. The author has studied successively the glycols, the oxides of ethylene and the halohydrins.Among the
 -glycols he finds that the primary-secondary and primary-tertiary, after dehydration, rearrange themselves into aldehydes with only a shifting of the position of the hydrogen atom; for example, RCH(OH)CH2OH
-glycols he finds that the primary-secondary and primary-tertiary, after dehydration, rearrange themselves into aldehydes with only a shifting of the position of the hydrogen atom; for example, RCH(OH)CH2OH  RCH=CHOH RCH2COH; bi-secondary into ketones with only the migration of the hydrogen atom, e.g., MeCH(OH)CH(OH)Me
RCH=CHOH RCH2COH; bi-secondary into ketones with only the migration of the hydrogen atom, e.g., MeCH(OH)CH(OH)Me  MeCH2COMe, or into aldehyde, with wandering of the phenyl group, provided this group is attached to the carbon atom carrying hydroxyl, which does not enter into the dehydration, thus, MeCH=C(OH)Me and PhCH=C(OH)Me, isomerize without migration of hydrocarbon group. PhCH=C(OH)Ph isomerizes with migration of a hydrocarbon group. In the case of the bi-tertiary the rearrangement cannot take place without a wandering of a hydrocarbon radicle. All the oxides of ethylene, except the tetra-substituted derivatives, isomerize with the simple migration of the hydrogen atom.
MeCH2COMe, or into aldehyde, with wandering of the phenyl group, provided this group is attached to the carbon atom carrying hydroxyl, which does not enter into the dehydration, thus, MeCH=C(OH)Me and PhCH=C(OH)Me, isomerize without migration of hydrocarbon group. PhCH=C(OH)Ph isomerizes with migration of a hydrocarbon group. In the case of the bi-tertiary the rearrangement cannot take place without a wandering of a hydrocarbon radicle. All the oxides of ethylene, except the tetra-substituted derivatives, isomerize with the simple migration of the hydrogen atom.In the case of the isomerization of iodohydrins of aromatic nature into aldehydes and ketones, the migration of the phenyl group takes place when it is attached in the hypothetical intermediate substance to the carbon carrying the hydroxyl group; the same is true of the magnesium derivatives of the halohydrins.
Experimental
Unsymmetrical methylphenylethyleneglycol, PhMeC(OH)CH2OH, can be made by the action of barium hydrate or carbonate in the presence of water on the dibromide of methovinylbenzene, or from methylmagnesium iodide and benzyl carbinol, m. 42-43°C, b26 160-162°C. Heated with dilute H2SO4 it gives hydratropic aldehyde. Unsym. methyl-p-tolylethyleneglycol, MeC6H4COHMeCH2OH, m. 36°C, b15 175-80°C, obtained in a similar manner to the preceding compound, by boiling with dil. H2SO4 gives methylhydratropic aldehyde, b730 219-221°C. Unsym. diphenylethyleneglycol, Ph2C(OH)CH2OH, from phenyl magnesium bromide and ethyl glycollate, m. 122°C. Boiled with dil. H2SO4, it gives diphenylacetaldehyde, b15 170-175°C. Symmetrical methylphenylglycol (See Fincke, Ber., 17, 710) gives phenylacetone.
The oxide of styrene shows a remarkable stability in the presence of hydrolytic agents, since it does not give phenylacetaldehyde with H2SO4 and HNO3 or AgNO3; the oxide of methylvinylbenzene, on the other hand, isomerizes very rapidly by heating with dil. H2SO4.
p-Methoxystyrolene, obtained by the method of Klages (Ber., 36, 3592) gives with mercuric oxide and iodine, methylphenylacetaldehyde, b. 255-256°C, d0 = 1.140, oxime, m. 121°C (Bouveault, Compt. rend., 135, 41), semicarbazone, m. 181-182°C. On reduction with zinc and acetic acid, the aldehyde gives the acetate of p-methoxyphenylethanol, b11, 156-7°C, d0= 1.101.
Phenylpropylene, by passing through the intermediate iodohydrin, gives hydratropic aldehyde, whose oxime is a liquid, b7 124°C. Reduced with zinc and acetic acid, the aldehyde gives the acetate of hydratropic alcohol, from which, by saponification, the alcohol may be obtained, b14 113-4°C. Phenylmagnesium bromide, with the aldehyde, gives the alcohol PhCHMeCHOHPh. From the iodohydrin of phenylpropylene one obtains phenyl-1-dimethylamino-2-propanol-1 (Fourneau, J. pharm. chim. [6], 20, 488), indicating the constitution of the iodohydrin. Methyl-3-phenylbutylene, obtained by dehydration of the alcohol, PhCHOHC2CHMe2, gives isopropylphenylacetaldehyde, b. 215-220°C, semicarbazone, m. 140°C. The iodohydrin of methylphenylglycol, PhMeCOHCH2I, gives phenylacetone. This ketone with phenylmagnesium bromide gives
 ,
, -diphenylpropylene (Klages, Ber., 35, 2648).
-diphenylpropylene (Klages, Ber., 35, 2648).n-Propylstyrolene, obtained from
 -propylcinnamic acid, which in turn was made from the ethyl ester, formed by the action of phenylbutanone in the presence of magnesium or ethyliodacetate, gives, through its iodohydrin, benzylpropylketone. The n-propylstyrolene gives a liquid dibromide.
-propylcinnamic acid, which in turn was made from the ethyl ester, formed by the action of phenylbutanone in the presence of magnesium or ethyliodacetate, gives, through its iodohydrin, benzylpropylketone. The n-propylstyrolene gives a liquid dibromide.Diphenylmethylcarbinol, Ph2COHMe, m. 80-81°C (Tiffeneau, Bull. soc. chim. [3], 27, 292; Klages, Ber., 25, 2646; Masson, Compt. rend., 135, 533) by distillation at the ordinary temperature, gives the unsaturated hydrocarbon, Ph2CH=CH2, which, through its iodohydrin, yield desoxybenzoin.
p-Tolylphenylmethyl carbinol, MeC6H4(Ph)C(OH)Me, is obtained as liquid from p-methylacetophenone. By repeated distillation at ordinary temperatures, it gives p-tolylphenylethylene, b. 285-286°C, b6 145-146°C The latter yields p-tolylacetophenone. Phenyl-2-butylene, through its iodohydrin, gives methylbenzylmethyl ketone, PhCHMeCOMe, b. 210-212°C, b23, 106-107°C.
Phenyl-2-n-amylene, PhMeC=CHCH2Me, gives a ketone which does not combine with sodium sulphite, b. 225-227°C. Semicarbazone, m. 188°C. Phenyl-3-n-amylene, PhC(Et)=CHMe, was made by dehydration of the diethylphenylcarbinol made by action of ethyl magnesium bromide on ethylbenzoate, b. 197-9°C, d0=0.9321. Through the iodohydrin it gives methylbenzylethyl ketone, MePhCHCOEt, b. 222-225°C, d0=0.982. Semicarbazone, m. 172°C. By the action of ethyl magnesium bromide on chloracetophenone, benzylethylketone is formed, semicarbazone, m. 146°C.
(Hive Addict)
07-14-03 10:23
No 447027
Symmetrical methylphenylglycol (See Fincke, Ber., 17, 710) gives phenylacetone
Fincke?
Ueber zwei isomere Phenylmethylglycole. I.
Th Zincke
Ber Deutsch chem Ges 17 (1884) 708-713 (../rhodium/pdf /p2p.phenylpr
Dirty old man
(Hive Addict)
07-29-03 15:44
No 450553
(Rated as: excellent)
To the phenols and phenol ethers with linear allyl and isoallyl (propenyl) chains,
-CH2-CH=CH2 (allyl), -CH=CH-CH3 (isoallyl),
which one finds so often in natural essences, there are two types of isomeric compounds with ramified pseudoallyl (methovinyl) and cycloallyl (trimethylene) chain,
-C(=CH2)-CH3 (pseudoallyl), -CH-CH2(>CH2) (cycloallyl),
whose presence in essential oils has not yet been proven and of which no represantative has been known until our research.
The importance of the allylic and isoallylic phenol ethers in pure and applied chemistry, as well as in plant physiology is so big that it seemed interesting to prepare and systematically explore their ramified isomers.
In reality, we have not yet prepared phenolethers with closed chain (cycloallyl) despite of one of us having done some private research in this direction on the most simple aromatic hydrocarbons. On the other hand, we have been able since some years to prepare at regular intervals a big number of phenol ethers with pseudoallyl chain, of which we like to report the complete investigation today.
Outside of public view, where we tended to place us with this work, our research presented at it's begin a very special interest; the synthesis of anethol and isoanethol, which we had just realised in conditions inhibiting molecular rearrangement, definitely confirmed the isoallyl structure; it followed that the remarkable transformation of these isoallyl derivates in hydratropic aldehydes under action of iodine and yellow mercury oxide was due to a molecular rearrangement.
Ar-CH=CH-CH3 --> Ar-CH(-CH3)-CHO
It has thus been extremely interesting to find out what happens to the ramified pseudoallyl chain under the same conditions; we observed that in this case one obtains an analogous rearrangement, but in the other direction, consisting of the straightening of the ramified chain to a linear chain.
-C(=CH2)-CH3 --> -CH2-CO-CH3
One of us, researching this rearrangement phenomenon, could show that it is due to the migration of the aromatic ring (aromatic transposition); we ourselves have observed that during this migration the chain rests attached to the same atom.
The preparation of phenol ethers with pseudo-allyl chain is done by reacting the corresponding esters with an excess of methylmagnesium iodide; we know that if this reaction is done with certain precautions, with only two organo metallic molecules one obtains the following tertiary alcohol:
r-CO2R' + 2IMgCH3 --> R-C(OMgI)(CH3)3 + IMgOR'
Indeed, we have thus obtained meta-anisyl-2-propanol, veratryl-2-propanol, vanillylpropanol, etc...
However Klages(1) showed that by using another molecule of methylmagnesium iodide and by distilling a part of the ether, one obtains the dehydration product of this tertiary alcohol; however since some phenol ethers polymerise easily, it seemed preferable to operate after the recommendations of Delange (2) using a big excess of methylmagnesium iodide (4 or 5 molecules) and to hydrolise by slightly acidic ice water without prior distillation. With the pseudo-allyl derivates one always obtains a small amount of dimers.
We applied this method to monophenolic (anisic and cresotic series) and diphenolic (vanillic, veratric, piperonylic) esters.
The physical constant of the thus prepared pseudoallylic ethers have been found to lie between those of the corresponding allyl and iso-allyl derivates; we have collected the most important ones in the following table.
The boiling points measured at atmospheric pressure are about 5 or 6° higher than those of the allyl isomers and 9 to 10° lower than those of the isoallyl derivates.
| bp | Anisol | Gaiacol | Veratrol | Methylene pyrocatechol |
| Allyl | 215-216° | 252° | 248-249° | 238° |
| Pseudo-allyl | 222° | 257-258° | 252-254° | 238-239° |
| Iso-allyl | 233° | 267° | 263° | 246-248° |
The densities of the pseudoallyl compounds, brought to 15° by calculation, are nearly exactly between the densities of the non-ramified isomers.
| Densities | Anisol | Gaiacol | Veratrol | Methylene pyrocatechol |
| Allyl | 0.0755 | 1.0715 | 1.037 | 1.107 |
| Pseudo-allyl | 0.985 | 1.0832 | 1.048 | 1.119 |
| Iso-allyl | 0.995 | 1.092 | 1.0605 | 1.123 |
The refraction indices of the phenolic ethers with pseudo-allyl chain lie 20 thousandth parts above the one of the corresponding allyl derivates and 15 thousandth parts below the one of the isoallyl derivates; however, if these phenolic ethers contain a free phenolic function, like in eugenol, iso or pseudoeugenol (allyl, isoallyl, pseudo-allylgaiacol) the spreads are less notable.
| Refraction indices | Anisol | Gaiacol | Veratrol | Methylene pyrocatechol |
| Allyl | 1.5236 | 1.5439 | 1.5373 | 1.5423 |
| Pseudo-allyl | 1.5445 | 1.5595 | 1.5560 | 1.5619 |
| Iso-allyl | 1.5615 | 1.5688 | 1.5720 | 1.5763 |
The chemical properties of the pseudoallyl phenol ethers more specifically resembles those of the corresponding iso-allyl compounds; like those they can be hydrogenated by sodium and absolute alcohol whereas in the same conditions the allyl derivates cannot be reduced.
When treated with IOH, they give iodohydrines, which are subject to a molecular rearrangement of the same type as for the isoallyl compounds, but in the opposite direction.
ArC(=CH2)-CH3 --> ArC(OH)(CH2I)(CH3) --> Ar-CH2-CO-CH3
ArCH=CH-CH3 --> Ar-CHOH-CHI-CH3 --> Ar-CH(-CHO)-CH3
On the other hand, the allyl-phenol ethers, while fixing IOH, are not subject to molecular rearrangement (Bougault (1)).
When treated with dry potassium hydroxide, these iodohydrins eliminate HI without rearrangement and give asymmetric ethylene oxides which easily isomerise to hydratropic aldehydes.
The pseudoallyl phenol ethers spontaneously oxidise with formation of trioxymethylene and acetophenones:
ArC(=CH2)-CH3 --> Ar-CO-CH3 + CH2O
The dimers of the pseudoallyl phenol ethers are very likely symmetric derivates of tetramethylene
Ar(CH3)>C<(CH2)2>C<Ar(CH3)
their molecular weights, determined by cryoscopy corresponds to the above formula; they do not fixate bromine and do not decolorise a cold solution of permanganate.
Nomenclature of phenolic ethers with C3H7 chain
The most rational nomenclature would be to denote the aromatic radical by it's conventional name (anisyl, veratryl, piperonyl) and let follow the name of the side chain using new nomenclature; one would thus obtain: 1-anisyl-1-propene (anethol), vanillyl-2-propene (eugenol), 2-veratryl-1-propene (pseudomethyleugenol), piperonyl-cyclopropane (trimethylene)
CH2O2-C6H3-CH<(CH2)2;
one sees that such a nomenclature does not capture the relation between the side chains of those four types of compounds. Thus if one wants to primarily point out the nature of the side chain, it is preferable to use an totally arbitrary nomenclature, which keeps the designation of the known isomers: eugenol and isoeugenol, safrole and isosafrole, estragol and isoestragole (anethol), while the new isomers are named with the prefixes pseudo for the C(=CH2)-CH3 chain and cyclo for the trimethylene chain; on can also, like we did until now (mainly for the representation of those compounds in tables) take as basis the term allylic and derive the expressions isoallylic (propenylic), pseudoallylic (methovinylic), cycloallylic (trimethylenic)
In the three following memoirs, we will explore the pseudoallylic phenol ethers one at a time: 1. the anisic and homoanisic series; 2. the cresotic series (synthesis of thymols); 3. vanillic and piperonylic series (pseudoeugenol and pseudosafrole).
(Hive Addict)
07-29-03 15:48
No 450554
(Rated as: excellent)
Anisol has three corresponding monosubstituted pseudoallylic derivates; these are prepared using the ethers of the ortho,
meta, and parabenzoic acids. Just like ortho and meta-anethole, ortho and meta-pseudoallylanisol are liquids; the para derivate, like anethol is a solid and melts at 32°.
Their different constants are summarised in this table:
| CH3O-C4H6-C(=CH2)-CH3 | boiling point | density | refraction index |
| orthopseudoallylanisol | 198-199 | 0.983 at 21° | 1.5315 |
| metapseudoallylanisol | 216-217 | 0.9878 at 21° | 1.5417 |
| parapseudoallylanisol | 222 | melts at 32° | 1.5417 |
The properties of those pseudo allylanisols are those that we have pointed out for all phenol ethers with pseudo-allylic chain. Reaction of iodine and yellow mercury oxide with two of those isomers gave molecular rearrangement to meta- respectively paramethoxy phenylacetones and we could confirm the observation made be one of us, namely that the side chain rests attached to the same atom of the aromatic ring. Indeed these ketones give m.- and p.-oxybenzoic acids on chromic oxidation.
This compound was obtained either by methylation of the corresponding phenol or by direct action of methyl magnesium iodide on methyl ortho-methoxy benzoate.
a) Methylation of o.-pseudoallylphenol. - O.-pseudoallylphenol was obtained by treating methyl salicylate with 3 molecules IMgCH3; this phenol boils at 204°, d0=1.0528. Methylated in potassium hydroxide solution by methyl sulfate, it gives o.-pseudoallyanisol in quantitative yields.
b) Reaction of IMgCH3 with methyl methoxybenzoate (methylsalicylic ether). - Controlled reaction of two molecules of the organo magnesium compound with methyl salicylic ether gives dimethyl-o.-anisylcarbinol, melting at 15° and boiling at 239°. Using three molecules of methyl magnesium iodide and maintaining at reflux for some hours directly gives o.-pseudoanisol. It boils at 198.199° at atmospheric pressure. Density at 21°=0.983; nD19=1.5315; RM found 46.6, calculated 46.^3.
Analysis. - Subst., 0.3041g; CO2, 0.9074g; H2O 0.2225g
Found: % C, 81.37; H, 8.12 - Calculated for C10H12O: % C, 81.08; H, 8.10
Hydrogenation. - 25 gr. pseudoestragol are reduced by 20 gr. sodium and 200 gr. absolute alcohol; the crude product is shaken with a KMnO4 solution, steam distilled, dried and rectified; the main fraction (9 gr.), boiling at 194-196° is o.-isopropylanisol. D0=0.9655; d16=0.9532; nD15=1.50891; RM found 46.95, calculated 46.52.
Analysis. - Subst., 0.3409g; CO2, 0.9985g; H2O, 0.2814.
Found: % C, 79.88; H, 9.17. - Calculated for C10H14O: % C, 80; H, 9.33
Demethylation is effectuated by slightly refluxing with HI for 12 hours. Usual workup gives orthoisopropylphenol OH-C6H4-CH(CH3)2 boiling at 207-208° at atmospheric pressure and melting at 9-10°. Alcoholic solution gives green coloration with iron perchlorate.
It was prepared via the metaoxybenzoic ester. In order to obtain the latter, the methyl ether of paracresol OCH3-C6H4-CH3 was oxydised by permanganate and the resulting metamethoxybenzoic acid was esterified with ethyl alcohol.
Ethyl m.-methoxybenzoate (bp 250-252°, d18=1.100) was reacted with IMgCH3; with 2 molecules of this reagent the corresponding tertiary alcohol, dimethyl-m.-anisyl-carbinol, bp 242°@770mm is obtained, which crystallises in pet. ether giving needles melting at 34°.
Analyse. - Subst., 0.2318g; CO2, 0.6146g; H2O, 0.1822g.
Found: % C, 72.32; H, 8.78 - Calculated for C10H14O2: % C, 72.28; H, 8.43
With three molecules of IMgCH3 the dehydration product of this alcohol, m-pseudoallylanisol, is obtained. This alcohol boils at 216-217°@770 mm.; d0=1.0041, d18=0.9901. It adds 2Br and rapidly decolorises permangante at low temperatures.
Hydrogenated with sodium and alcohol it gives meta-isopropylanisol, boiling at 210-211° at atmospheric pressure, d0=0.9642; demethylated by HI, the latter gives m-isopropylphenol, melting at 26°.
Potassium permanganate transforms m.pseudo-allylanisol in m.methoxyacetophenone boiling at 240° at atmospheric pressure.
With HgO + I m.-methoxyphenylacetone, not yet described, is obtained; it boils at 258-260°, d0=1.0812 and the semicarbazone derivate melts at 175°. The constitution of this phenylacetone is proven by the action of I + NaOH, giving m.-methoxyphenylacetic acid and iodoform; further it gives m.-methabenzoic acid, melting at 109°, by chromic oxidation.
It was prepared by reacting methylmagnesiumiodide (2 or 3 molecules) with methyl or ethyl anisate (1 mol.) for one or two hours on steam bath; after hydrolysis with acidic ice water, the product is treated for one hour with alcoholic potassium hydroxide, which saponifies excess anisic ester; then, the neutral residue is steam distilled; using two molecules organo magnesium compound, pseudo estragol was directly obtained with 50% yield per weight of anisic ester, without accounting for the anisic acid recovered by acidifying the alcaline liquors; from the fraction which is not volatile with steam spontaneously crystallises a dimer melting at 58°; with three molecule methylmagnesium iodide, the yield of pseudoestragol was much worse; and instead of the latter the oxide of the corresponding alcohol
CH3O-C6H4-C(CH3)2-O-(CH3)2C-C6H4-OCH3
boiling at 260-270°@31mm is formed, which doesn't crystallise when cooling to 15°.
Analyses. - Subst., 0.3135gr; CO2, 0.8941gr; H2O 0.2667gr.
Found: % C, 77.77; H, 9.4 - Subst., 0.3517gr; CO2, 0.9954gr; H2O, 0.2836gr. Founds: % C, 77.18; H, 8.95. - Calculated for the oxide C20H26O3: % C, 76.43; H, 8.28.
This oxide, treated with steam distillation with 10% sulfuric acid transforms partially into crystallised pseudo-estragol.
The recrystallised pseudo-estragol melts at 32°; it distills at 222° at atmospheric pressure; on supercooling nD21=1.5423.
Reduction. - 42gr. pseudo-estragol were reduced by sodium in absolute ethanol; after multiple rectifications of the obtained product, 28gr. para-isopropylanisol, boiling at 210-212° were obtained, d0=0.9638, d16=0.9518, nD16=1.5088.
Analysis. - Subst., 0.2947gr; CO2, 0.8621gr; H2O, 0.2266gr.
Found: % C, 79.75; H, 8.92. - Calculated for C10H14O: % C,80.0; H, 9.33.
Our attempts to demethylise by gaseous HBr, hot or cold, at atmospheric pressure were futile; the demethylation of 20gr. para-isopropylanisol by HI gave 8gr. p.-isopropylphenol OH-C6H4-C3H7, melting at 59-60° and distilling at 228-229°; the benzoyl derivate obtained by treatement of the sodium solution with benzoyl chloride melts at 70-71° after recrystallisation in alcohol.
Oxydation. - Pseudo-estragol is transformed by permanganate in p.-methoxyacetophenone, melting at 39°. The pseudo-estragol is spontaneously oxidised with formation of trioxymethylene, which can be identified by the strong smell. The quantity formed is big enough to be chemically characterised by the tongue. (
Pseudo-estragol (2 mol) is dissolved in ether saturated with water, first yellow mercury oxide (1 mol) and then about 4 atoms of iodine are added; the etheric solution is filtered, washed with a solution of KI with a few drops of bisulfite and finally with pure water; after drying on sodium sulfate, the ether is rapidly removed by vacuum without heat; the residue is the iodohydrine pictured above.
Reaction with potassium hydroxide: formation of the ethylene oxide isomerising into aldehyde. Dry potassium hydroxide, which is directly added to the etheric solution obtained above before removing the ether, transforms the iodohydrine into the corresponding ethylene oxide: pseudo-estragol oxide (I); it boils at 130-135°@12mm.; but it can't be distilled at atmospheric pressure without being transformed into p.-methoxyhydratropic aldehyde (II).
(I) CHO3-C6H4-C(CH3)-CH2>O --> CHO2-C6H4-CH(-CH3)-CHO (II)
This aldehyde boils at 135°@15mm. ; it was described by Bougault (1), who prepared the oxime, which melts like ours at 96°. We also prepared the semicarbazone, which melts at 199-200°. After recrystallisation in benzine the melting point increases to 207-208° (on block).
Mr. Balbiano and Mr. Paolini (2) found the semicarbazone of p-methoxy hydratropic aldehyde to melt at 134°; we noted that, from the pure aldehyde, regenerated from the polymer described below by distillation at atmospheric pressure, one indeed obtains a semicarbazone melting at 134°; anyway, when the crude semicarbazone is extracted with benzene, one obtains a sparsely soluble residue, melting at 207-208°, and a solution from which crystallises the semicarbazone melting at 135°. Furthermore we have decomposed 1.5gr. of semicarbazone melting at 207° with H2SO4 and steam distilled; the obtained product colors Schiff reagent, has the typical p.-methoxyhydratropic aldehyde and gives a semicarbazone, which is separated into two fractions by benzene, one melting at 135° and one melting at 207°.
When kept for some time, p.-methoxyhydratropic aldehyde transforms into a polymer, which can be purified by crystallisation in alcohol or benzene; the melting point is 103-104°C on sulfuric acid and 106° on Maquenne block.
Analysis. - Subst., 0.2122gr; CO2 0.5708gr; H2O, 0.1432gr
Found: % C, 73.36; H, 7.49. - Calculated for C10H12O2: % C, 73.17; H, 7.31.
Cryoscopy. - By cryoscopy we found a mean molecular weight of M=431; for a trimer, the molecular weight would be 492. By simple distillation at atmospheric pressure, this polymer is retransformed into pure monomeric aldehyde.
Reaction with AgNO3: rearrangement into anisic acetone. - When the etheric iodohydrine solution above is slowly added to a heavily stirred concentrated silver nitrate solution, immediately white crystalline silver iodonitrate precipitates; the washed etheric solution, shaken with bisulfite gives big amounts of bisulfite adduct, which is easily decomposed by hot water, even when only purified by multiple washings with ether; the decomposition of this bisulfite adduct by heating on water bath with water or sodium carbonate solution gives the acetone CH2O-C6H4-CH2-CO-CH3. boiling at 264° and identical to anisylacetone discovered by Bouchardat and Tardy(1) in anis and fennel essences; the oxime melts at 72°, but Hoering (2) proved that it's a mixture of two isomeric oximes, melting at 78-79° (alpha) and 61-62 (beta) respectively; the semicarbazone melts at 182° (2). This ketone gives iodoform ant p.-methoxy phenylacetic acid, melting at 86°, when reacted with iodine and sodium carbonate.
In the majority of cases, when preparing pseudo-estragol, from the products non volatile with steam crystallises a solid compound, which can by purified by crystallisation in alcohol. This pseudo-estragol dimer, which has probably the following composition:
CH3O-C6H4-(CH3-)C<(CH2)2>C(-CH3)-C
The union of the two monomeric molecules must happen by the double bond, since the dimer does not react with bromine and does not decolorise cold potassium permanganate solution. It melts at 58° and distills at 210-215°@15mm; when one tries to distill at atmospheric pressure, one notes that the thermometer in the boiling liquid shows 350°, but the liquid which distills comes at about 220-230°; indeed the heat partially transforms this dimer into pseudo-estragol, with formation of a small amount of isopropylanisol.
This derivate was obtained via ethyl paraethoxybenzoate (bp 274-275°, 148-149°@14mm, d21=1076). An etheric solution of 100gr. of this ester is added to methylmagnesium iodide, prepared from 30 gr. magnesium. The crude product (80 gr.) is treated with alcoholic potassium hydroxide, which regenerates 9.5gr. p.-ethoxybenzoic acid; the residue is steam distilled; 8gr. slowly crystallising dimer, non volatile with steam, and 55gr. product, which is vacuum fractionated are thus obtained; 35gr. distill at 110-115°@13mm and rapidly crystallise; further purification is done by crystallisation from alcohol.
Analysis. - Subst., 0.3253gr; CO2, 0.9725gr; H2O, 0.2839gr.
Found: % C, 81.54; H, 9.66. - Calculated for C17H24O: % C,81.40; H, 8.64.
p.-pseudo-allylphenetol melts at 27-28°; it boils at 113-114°@13mm and at 231-232° at atmospheric pressure (corr. 234-235°), d31=0.969,nD21=1.540536.
The pseudoallylphenetol dimer melts at 74°; when it is heated for distillation at atmospheric pressure, the thermometer in the liquid shows 365-370°, whereas small quantities of monomer come at 232°.
Hydrogenated by sodium and absolute alcohol, p.-pseudoallylphenetol gives p.-isopropylphenetol boiling at 220°; d0=0.9464, d23=0.9286,nD34=1.4974; hydroiodic acid gives p.-isopropylphenol melting at 59-60°.
Yellow mercury oxide and iodine give the usual rearrangement with formation of ethoxy phenylacetone, boiling at 270-272° and melting at 41°.
(Hive Bee)
10-20-03 18:38
No 465776
(Rated as: good read)
From Ber. 60, 1050 (1927) the (I think) important steps:
In the first step they synthesice alpha-metylstyrene IUPAC: 2-Phenylpropene , in the next step they converted this with 83% yield to the Oxid IUPAC: 2-Methyl-2-phenyl-oxirane
The Oxid was converted by Hydration ( with aqueous HCl) to the corresponding Glykol (bad yield) IUPAC: 2 Phenyl-propane-1,2-diol
This Glykol was also synthesiced (with better yield) from Acetophenone.
With Oxid and Glykol than are following some isomerisations pp:
From Oxid
I.
The Oxid (on clay) is distilled with 91% yield of Methyl-Phenyl-acetaldehyd ( = Aldehyd I)
II.
The Oxid on Pumice with 50% H2SO4 is heated to 140-150° with 64% yield of
( =Aldehyd I)
III.
The Oxid with H2SO4 at –15° C yields 46% BMK
From Glykol
I.
The Glykol under CO2 Steam with 20% H2SO4 yields 80% Methyl-phenylacetaldehyd (=Aldehyd II)
II.
6 g Glykol ,12 g Oxalic Acid and 6 ml H2O are (water bath) heated (under CO2 Steam) and the yield is 82% BMK
III.
50% H2SO4 (50-55°) unter CO2 Steam. Aldehyd II und BMK with a ratio of 2:1
IV.
With H2SO4 at –15° yield 48% BMK
Aldehyd I
I .
Yields with concentrated ( –16°) H2SO4 48% BMK
III.
Yields with HgCl2 a Mixture from 90% Aldehyd and 10% BMK
Aldehyd II
II.
Yields with ( –16°) H2SO4 62% BMK
IV.
with HgCl2 80% BMK
They differ between Aldehyd I and Aldehyd II, but I think it`s Hydratopaldehyde with different impurities.
(Chief Bee)
06-07-04 00:14
No 511840
(Rated as: excellent)
As you can see in the articles above, both alpha-methyl-styrenes and allylbenzenes (like safrole) can be turned into their corresponding phenylacetones (P2P/MDP2P etc.) by making the iodohydrin followed by acid/base hydrolysis. Unfortunately the authors back then used iodine together with a huge amount of a mercury salt to prepare the iodohydrins, considerably lessening the convenience of this route. However, in the 100 years since the above articles were written, there has been considerable improvements made in the field of iodohydrin formation, and below I present the most important advances:
Iodohydrins and Epoxides from Olefins
J. W. Cornforth and D. T. Green
J. Chem. Soc. (C), 846-849 (1970) (../rhodium/pdf /iodohydrin.h
Abstract
Reaction of olefins with iodine in the presence of water and a suitable oxidizing agent such as iodic acid, or oxygen catalysed by nitrous acid, gives iodohydrins. These form epoxides readily on treatment with bases.
____ ___ __ _
Acetoxyiodination of Olefins: A New Method for the Preparation of trans-Iodohydrin Acetates
Michelangelo Parrilli, Gaspare Barone, Matteo Adinolfi and Lorenzo Mangoni
Gazzetta Chimica Italiana 104, 835-842 (1974) (../rhodium/pdf /alkene.aceto
Abstract
The reaction of 1-methyl-4-tert-butylcyclohexene and of 1-methylcyclohexene with I2 and KIO3 in AcOH has been shown to give mainly the acetoxy-iodides [Ib] and [IIb] and the acetoxy-iodides [IIIb] and [IVb], respectively. Therefore, it may be considered to be a new one-step method for the preparation of trans-iodohydrin acetates starting from olefins. Product distribution showed that in the first stage of the reaction the iodonium ions formation is reversible.
____ ___ __ _
An improved synthesis of iodohydrins from alkenes
M. Smietana, V. Gouverneur and C. Mioskowski
Tetrahedron Letters 41(2), 193-195 (2000) (../rhodium/pdf /alkene2iodoh
Abstract
A series of iodohydrins was prepared in excellent yields in a one-step procedure by treating the corresponding alkenes at -20°C with NIS in aqueous DME.
____ ___ __ _
The procedure in the last article requires the quite expensive compound N-Iodosuccinimide (NIS), but that can be made in high yield from sodium iodide and NCS (and presumably NBS as well), see the following article:
N-Chlorosuccinimide/Sodium Iodide: A Convenient Source of N-Iodosuccinimide (NIS)
Synthesis of trans-1,2-Iodoacetates from Alkenes
Yashwant D. Vankar and G. Kumaravel
Tetrahedron Letters 25(2), 233-236 (1984) (../rhodium /nis.al
Abstract
Equimolar amounts of N-chlorosuccinimide and sodium iodide in acetone are found to be a convenient source of N-iodosuccinimide using which trans-1,2-iodoacetates and ?-iodo carbonyl compounds have been prepared from olefins and enol silyl ethers respectively.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
06-07-04 21:12
No 512025
You say that P2P can be made "... from 2-phenyl-1,2-propandiol..." ?! I think you meant to say 1-phenyl, 1,2-propandiol.
But since you mentioned that P2P can be made from 1-phenyl, 1,2-propandiol let me just mention that a long time ago I posted an article that synthesized 1-phenyl, 1,2-propandiol from benzaldehyde and yeast!
use the search engine...
(Chief Bee)
06-07-04 21:33
No 512034
Yes, P2P can be made by acid treatment of 1-phenyl-1,2-propanediol, but that is well known (as this corresponds to the isosafrole glycol intermediate gotten in the peracid oxidation of isosafrole).
The novel thing here is the re-discovery that 2-phenyl-1,2-propanediol (alpha-methyl-styrene glycol) will also give P2P under certain conditions. See the partial translation of the article I found in Post 465776 (roger2003: "Ber. 60, 1050 (1927)", Novel Discourse)
From 2-Phenyl-propane-1,2-diol
I. The Glykol under CO2 Steam with 20% H2SO4 yields 80% Methyl-phenylacetaldehyd (= Aldehyd II)
II. 6 g Glykol, 12 g Oxalic Acid and 6 ml H2O are (water bath) heated (under CO2 Steam) and the yield is 82% BMK
III. 50% H2SO4 (50-55°C) unter CO2 Steam. Aldehyd II und BMK with a ratio of 2:1
IV. With H2SO4 at –15°C yield 48% BMK
The Hive - Clandestine Chemists Without Borders