04-13-04 09:46
No 500591
This is really frustrating... I have no idea how to aquire any more info on this exciting ACS conference abstract...
The Carroll Rearrangement: A Facile Entry Into Phenylacetone and Related Derivatives
Kirk L. Sorgi and Lorraine Scott
Department of Chemical Development, R. W. Johnson Pharmaceutical Research Institute, Spring House, PA. 19477
Abstracts of Papers of the American Chemical Society 200(2), 297-ORGN (1990)
Abstract
The Carroll rearrangement, a variant to the ester Claisen rearrangement, is a useful method for preparing
 ,
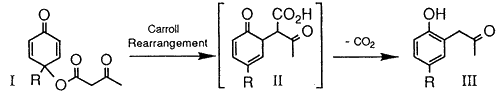
, -unsaturated ketones from allylic acetoacetates. The reaction has found limited action in synthetic organic chemistry due to the harsh thermal conditions normally needed to induce the [3,3]-sigmatropic rearrangement. While examining the chemistry involving allylic acetoacetate I, we encountered a mild (room temperature) Carroll rearrangement that afforded the phenylacetone derivative III via keto-acid II. Several examples showing the generality, scope, and limitations of the methodology will be described along with mechanistic considerations.
-unsaturated ketones from allylic acetoacetates. The reaction has found limited action in synthetic organic chemistry due to the harsh thermal conditions normally needed to induce the [3,3]-sigmatropic rearrangement. While examining the chemistry involving allylic acetoacetate I, we encountered a mild (room temperature) Carroll rearrangement that afforded the phenylacetone derivative III via keto-acid II. Several examples showing the generality, scope, and limitations of the methodology will be described along with mechanistic considerations.Carroll Rearrangement References
[sub]
http://themerckindex.cambridgesoft.com/T
http://www.chempensoftware.com/reactions
http://www.chem.ox.ac.uk/thirdyearcomput
The Hive - Clandestine Chemists Without Borders
(Hive Adickt)
04-13-04 10:24
No 500601
Beilstein turned up with thois one.
Wessely; MOCMB7; Monatsh.Chem.; 90; 1959; 713, 718.
Iff you have it online (i don't) it might be worth a look
(Newbee)
04-13-04 11:19
No 500609
ref. is Tetrahedron Letters, Vol. 36, No. 21, pp. 3597-3600, 1995.
"The Carroll Rearrangement:
A Facile Entry into Substituted Arylacetones
and Related Derivatives
Kirk L. Sorgi,* Lorraine Scott, and Cynthia A. Maryanoff
Chemical Development Department
The R. W. Johnson Pharmaceutical Research Institute
Spring House, PA 19477 USA
Abstract: Acetoacetates, easily prepared from substituted p-quinols, undergo a mild room temperature
Carroll rearrangement to afford substituted arylacetones and related derivatives in moderate to good
yields...."
Rhodium
If you want the pdf ask me by pm...
W S F - for a better world.
(Chief Bee)
04-13-04 13:21
No 500642
Thanks Kurupira! Here's the full article - It was way stranger chemistry than I imagined...
The Carroll rearrangement: A facile entry into substituted arylacetones and related derivatives
Kirk L. Sorgi, Lorraine Scott and Cynthia A. Maryanoff
Tetrahedron Letters 36(21), 3597-3600 (1995) (../rhodium/pdf /carroll.rear
Abstract
Acetoacetates, easily prepared from substituted p-quinols, undergo a mild room temperature Carroll rearrangement to afford substituted arylacetones and related derivatives in moderate to good yields.
The Hive - Clandestine Chemists Without Borders