(Hive Bee)
07-18-04 01:04
No 520044
(Rated as: good read)
Here is an intersting preparation of syringaldehyde using piperidine:
Tetrahedron: Asymmetry 13 (2002) 1799–1804
A solution of 3,4,5-trimethoxybenzaldehyde 10 (3.48 g, 17.7 mmol) in piperidine (35 mL) and water (35 mL) was heated under reflux for 48 h and the cooled mixture was poured into 4N aqueous hydrochloric acid. The mixture was extracted with ethyl acetate (3×50 mL). The combined organic layer was washed with 2N hydrochloric acid, water, dried over Na2SO4. The solvent was distilled off and the residue was flash chromatographed using petroleum ether and ethyl acetate (4:1, v/v) as eluent to afford syringaldehyde as a white solid (2.58 g, 80%). Mp 113–114°C.
(Hive Bee)
07-19-04 10:22
No 520297
Maybe this would be interesting in this thread......
Post 514141 (psyloxy: "3,4,5 precursors revisited", Chemistry Discourse)
Just hold on to the thread...that keeps us going
http://www.chiapaslink.ukgateway.net/
07-30-04 21:37
(Rated as: insignificant)
(Hive Addict)
07-31-04 01:02
No 522896
Actually, I think isoproscaline sounds quite interesting.
Haven't seen anything demethylated this way before, or then it is because of my memory. I guess this produces N-methylpiperidine and needs a substrate that is prone to demethylation.
fear fear hate hate
(Hive Bee)
09-03-04 12:41
No 529398
Every good bee knows that vanillin (via syringaldehyde) is best precursor for the 3,4,5 substitution pattern (escaline, etc.), hence the bromination of vanillin ortho to -OH has been a subject of much discussion at the hive. From vanillin you can go to plenty places in Pihkal, even MDA compounds if you like. However, for the 2,4,5 substitution pattern (MEM, ORTHO-DOT*) it would be essential to put the Br meta to OH in vanillin. It could bee done by first making the acethoxy ester, followed by bromination and treatment with HCl:
5-Bromo-4-formyl-2-methoxyphenol:
To the suspension of 4-acetoxy-3-methoxy-benzaldehyde (3.23 g, 16.7 mM) in KBr (6.67 g) and distilled water (80 mL) was added bromine (2.94 g, 18.4 mmol) dropwise. The reaction mixture was stirred for 10 h at room temperature and then subjected to filtration. The precipitate was suspended in 6 N HCl (80 mL) at 90 °C for 10 h. The reation mixture was then cooled and filtered and the resulting solid was dissolved in EtOAc and washed with saturated NaHCO3 solution. The organic layer was dried over MgSO4 and concentrated. The residue was recrystallized from EtOA-hexane to give 5-Bromo-4-formyl-2-methoxyphenol (3.58 g, 93% yield) as a colorless solid. Mp 174-175 °C (from hexanes, lit. mp 174-175 °C)
* Notice how Shulgin tries 2-TOM from 60 + mg where it has the makings of a great compound, he tries ORTHO-DOT at only 25 mg and gets vague activity. At higher levels it might be as dark compound as TMA-2, don't you think so?
(Chief Bee)
09-03-04 17:01
No 529473
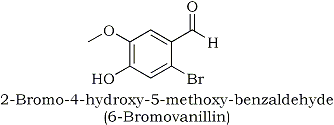
5-Bromo-4-formyl-2-methoxyphenol is a rather cryptic and inappropriate name for
this molecule - I even had to draw it to be able to identify its structure.
Its structure and proper IUPAC name follows here below:
The Hive - Clandestine Chemists Without Borders
(Stranger)
09-06-04 10:21
No 529913
What is strange is that the nitration of the same acetoxy vanillin give the 2-nitro compound, that is 2-nitro-3-methoxy-4-hydroxy benzaldehyde...
I wonder why, I thought it was the acetoxy which gave this orientation, but apparently not...
Captain: why dont you alkylate vanillin before the bromination, the product orientation is 2,4,5 in that case... and you dont need to generate the 4-acetoxy of the vanillin... any write up for this from GAA?
good luck with the methylmercaptan swap, hehe it will have quite a smell
so much dark compound, TMA-2
(Hive Bee)
09-07-04 00:40
No 530078
Yes, of course
So much darkness in so few miligrams... ;)
(Chief Bee)
09-29-04 05:05
No 533742
(Rated as: good read)
4-Desmethylmescaline
A. Brossi and S. Teitel
Org. Prep. Proced. Int. 1(3), 171-172 (1969)
4-Desmethylmescaline, a potential metabolite of mescaline which occurs as a minor alkaloid in Lophophora williamsii and Trichocerus pachanoi1, has been prepared from syringaldehyde by a multistep procedure2. A more convenient route is the facile conversion of mescaline by selective demethylation with mineral acid to afford 4-desmethylmescaline in 64% over-all yield. This procedure is another example of the preferential cleavage of the middle methoxyl that has been demonstrated3,4 for various alkaloidal systems containing three neighboring methoxy groups.
Experimental
A solution of 8 g. (32.4 mmole) of mescaline hydrochloride in 100 mL of 20% hydrochloric acid is refluxed for 2 hrs. and evaporated at 40°C under reduced pressure. The residual oil is crystallized from 100 ml. of ethanol to give 2.4 g of 4-desmethylmescaline hydrochloride: mp 249-250°C. λEtOHmax sh 230 nm (6500), 272 (1280); nmr (DMSO-d6, 60-Mc, tetramethylsilane) δ s 2.93 (2CH2), 3.76 (2CH3O), 6.53 (2 aromatics), 8.23 (OH and NH3); tlc (silica gel G, developed with 100:10:5 EtOAc-MeOH:NH4OH for 11 cm. and detected with Dragendorff's reagent), Rf 0.40; identical in m.m.p., uv, nmr and tlc to an authentic sample2. Mescaline hydrochloride (4 g.) is recovered by evaporation of the filtrate followed by crystallization from 100 ml. of acetonitrile. The overall yield of 4-desmethylmescaline hydrochloride is thus 64%.
References
[1] S. Agurell and J. Lundström, Chem. Commun., 1638 (1968)
[2] F. Benington, R. D. Morin, and L. C. Clarke, Jr., J. Amer, Chem. Soc., 76, 5555 (1954) Post 523232 (Rhodium: "Synthesis of Escaline and Homosyringylamine", Novel Discourse)
[3] A. Brossi, J. Van Burik, and S. Teitel, Helv. Chim. Acta, 51, 1965 (1968)
[4] A. Brossi, J. O'Brien, and S. Teitel, Helv. Chim. Acta, in press.
The Hive - Clandestine Chemists Without Borders
(Über-Führer die Ironie)
10-05-04 02:44
No 534515
Yes, but how would you alkylate the -OH w/o alkylating the amine too?
(Über-Führer die Ironie)
10-05-04 16:39
No 534598
(Rated as: excellent)
Actually, I think isoproscaline sounds quite interesting.
Yeah moo, I agree, it sounds very interesting, I would however like to taste the sec-butoxy analog, I don't think it has yet been made so I'm working on it.
For instance:
[Escaline: DOSAGE: 40 - 60 mg. DURATION: 8 - 12 h.]
[isoproscaline: DOSAGE: 40 - 80 mg. DURATION: 10 - 16 h.]
[proscaline: DOSAGE: 30 - 60 mg. DURATION: 8 - 12 h.]
[secbutoxy analog would hence probably have the same dosage/duration as proscaline, but it would probably halt at this lenght as receptor won't tolerate further homologation, see for instance the drop in potency with buscaline compared to proscaline (possibly the branched para-[3-penthoxy] would also show some activity, but it's hard to predict).]
Anyways, to the point of the post, this paper might interest you, notice how bromine on para shows increasd activity in sheep just like higher alkyloxy homolgs do, I think this could bee the study that Shulgin comments on in the DESOXY entry in Pihkal*:
Lipophilicity and serotonin agonist activity in a series of 4-substituted mescaline analogs
David E. Nichols, Donald C. Dyer;
J. Med. Chem.; 1977; 20(2); 299-301.

*: A mescaline analogue with a bromo atom in place of the 4-methoxyl group is an analogue of mescaline in exactly the same way that DOB (a very potent am-phetamine) is an analog of TMA-2 (the original trisubstituted amphetamine). This analogue, 3,5-dimethoxy-4-bromoamphetamine, has been found to be a most effective serotonin agonist, and it is a possibility that it could be a most potent phenethylamine. But, as of the present time, it has never been assayed in man.
http://www.city-net.com/~mbt/pamithb.htm
(Newbee)
10-05-04 20:22
No 534625
Captain america couldn't you just pick your base carefully, as their will be a fair bit of difference between the pKa of a phenolic proton and that of an amine's.
(Chief Bee)
10-05-04 20:47
No 534631
That is inconsequential, as only phenols need to be deprotonated to their corresponding anions for alkylation to proceed, the only requirement is that they are present as their free base.
An alternative to the use of a protection group on the nitrogen is to use the Mitsunobu Reaction: Post 506741 (Rhodium: "O-Methylation of Phenolic Phenethylamine", Chemistry Discourse)
The Hive - Clandestine Chemists Without Borders
(Über-Führer die Ironie)
10-06-04 03:20
No 534656
That is inconsequential, as only phenols need to be deprotonated to their corresponding anions for alkylation to proceed, the only requirement is that they are present as their free base.
Text in red - I know and agree with, but then I don't understand what you meen...
http://www.city-net.com/~mbt/pamithb.htm
(Chief Bee)
10-06-04 11:44
No 534714
I said that phenols requires basic conditions to be alkylated, while amines can be alkylated under either neutral or basic conditions (in response to the post by Disciple). The link to the Tyrosine O-alkylation article answers your question.
The Hive - Clandestine Chemists Without Borders