06-02-02 21:26
No 316869
"While trying to find out more info I bumped into an interesting procedure on rhodium, which involves making it into Indoleacetaldehyde with sodium hypochlorite (ie bleach) at a 90% yield ( ../rhodium /indolea
Shitloads better yield, and easy peasy precursors...
Then you just dimethylamine, Pt catalyst and hydrogen and voila!!!
Dimethylamine is not watched, so it should be fairly easy to get from chem suppliers (of course best to get a friend to order...) Platinum catalyst might seem expensive, but it can be reused more than almost any other catalyst. Hydrogen is easy to generate via aluminium foil and hydrochloric acid.
This method looks to me like it is the best, cheapest and most efficient method to date.
1kg (4.9mol) of tryptophan is worth $300, which makes ~700g (4.41mol) of indole-3-acetaldehyde, which makes (at a 90% yield) 747g DMT. Probably less than $1000. And no real need to use glassware either - The first reaction could be done in a big glass jar, and the second reaction again in a big glass jar with mods to pipe in and bubble H2 and safely divert the H2 away from your airspace... I will put together a full procedure shortly, giving molar quantities and procedures."[orange]
Somebody posted this at DMT world. I have not been able to find this on rhodiums site, maybe I am just retarded or something. Anybody have any comments or experience with this procedure? Will it work? Seems very very simple if it is possible, with good yeilds as well.
Any input would be apprecieated
(Master Searcher)
06-02-02 21:39
No 316875
Catalytic hydrogenation doesn't work.
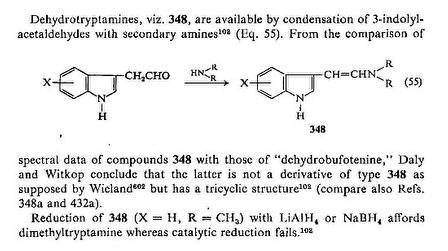
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Master Searcher)
06-02-02 22:50
No 316897
I first posted it here, but the image doesn't show up anymore. Post 299513 (PolytheneSam: "Holy grail?", Tryptamine Chemistry)
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Stranger)
06-03-02 01:17
No 316937
Ok thanks Psam;) But after reading that post I am left with the same question that zed is- can indoleacetaldahyde be reduced with dimethylamine in any other way? NaCNBH3 perhaps?
I must say that I have a very limited elementary understanding of chemistry so am am hoping that any wiser bee's thne myself could give me some input here.
(Master Searcher)
06-03-02 01:33
No 316945
The last line of the reference here Post 316875 (PolytheneSam: "Catalytic hydrogenation doesn't work.", Tryptamine Chemistry) says LiAlH4 and NaBH4 works. Also see Post 299552 (PolytheneSam: "Electrochemical reductive amination", Novel Discourse)
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Hive Bee)
06-03-02 05:29
No 317036
Delysid, reducing the imine via Al/Hg would probably work. This reduction is non-acetic, and works pretty well for related species. It doesn't require pricey and watched hydride reagents, just Aluminum foil, a small amount of Mercuric Chloride, and ordinary solvents.
The problem that concerns many of the bees, is the practical production of Indole Acetaldehyde in useful quantities. In the proceedure you mention, a large volume of solvent is required per gram of aldehyde recovered.
Hypochlorite is no joke as an oxidizing agent, it tears things up. This is a general problem with oxidations. Hard to stop.
Hence, my suggestion that it might be easier to produce large quantities of aldehyde oxime, via reducing the nitro-styrene analog produced from Indole-3-carboxaldehyde and nitromethane.
Alternately, DMT production from tryptophol is promising, and even simpler. Try the string on transamination, starting with Post 281536 (PolytheneSam: "Transamination idea", Tryptamine Chemistry).
Or, here is a low tech approach (theoretical):
Phalarius grasses are common. Cut (and blanch...if required)the grass of your choice. Blanching (dipping in boiling water)destroys enzymes that in some grasses might convert DMT to 5-Me DMT.
Dry the grass. Role it into giant spliffs (or pack it in stove pipe etc.)and use your shop vac. etc. as a vacuume source to "smoke" the spliffs like giant cigarettes.
Trap the tars in a filter or condensor system before the Spliff (or pipe) smoke reaches your vacuume source.
Extract or vacuume distill the product you desire, from the concentrated tars.
A few bales of Phalarius grass processed in this manner might produce an impressive amount of DMT.
Low tech, and it could keep your barn warm too. Of course, I'm not actually suggesting that anyone do this. But it is an interesting idea.
(Pioneer Researcher)
06-03-02 20:40
No 317217
Yes, it is a good way, and seems to work, but although it has been discussed here, nobody seems to have checked it. The problem I see is the big volumes used ot convert tryptophan to the aldehyde. I've tried a couple of procedures trying to make this rxn better but have failed.
(Hive Bee)
06-04-02 00:14
No 317256
Thanks Foxy!
Post 231988 (foxy2: "3-(2-Bromoethyl)indole Synthesis", Tryptamine Chemistry)
No barbed wire can cage a bee.
(Official Hive Translator)
06-04-02 12:20
No 317464
.......you could always reduce that IAAldehyde to tryptophol w/Al(OiPr)3 in a good yield and condense it w/NH(CH3)2 (or other amine of your choice) over Raney Ni, also in excellent yield
Antoncho