05-05-01 17:12
No 189415
A Base-Catalyzed Domino-Isomerization-Hydroamination Reaction - A New Synthetic Route to Amphetamines
Christian Hartung; Claudia Breindl; Annegret Tillack; Matthias Beller
Tetrahedron 56(29), 5157-5162 (2000)
Abstract
An efficient synthesis of pharmaceutically interesting amphetamines by a base-catalyzed domino-isomerization-hydroamination reaction is presented. Starting from allylbenzene and various primary or secondary amines, the basic structural pattern of amphetamines is synthesized directly in yields of up to 91% in the presence of catalytic amounts of n-butyllithium.
--psyloxy--
(Hive Bee)
05-05-01 20:30
No 189454
This was first dicussed a while ago (and then I bought it up again more recently). You must use benzylmethylamine and then reduce off the benzyl group to get your meth. I'm kinda curious about the bioactivity of the compound with the benzyl goup though.
something for your mind.......
(Master Whacker)
05-06-01 03:15
No 189490
Hey D_A,
Just wanted to let you know that many moons ago swim did quite a few reductive aminations of straight P2P with methylbenzylamine. To get the highest yields, it was found that the methylbenzylamine and P2P should be condensed first in boiling toluene using a Dean-Stark trap for water removal. After the theoretical amount of water was collected the resulting toluene soln of imine was rotovaped leaving a fairly viscous yellow oil. Catalytic hydrogenation in EtOH/HCl with 10% Pd/C produced about 85% yields of amazing n-methyl amine.
One day when Ritter was really board he dumped some NaBH4 into an ethanol solution of the amine to make N-Benzyl,N-Methyl amphetamine. Material was just about completely inactive (in SWIMS system anyway).
(Newbee)
02-17-02 23:07
No 270986
hi ritter,
excuse otto for being stupid, did you use gaseous hydrogen at room temperature? otto tried somewhat similar procedure on tmp2p, but in MeOH without acid at RT. the amine used was a-methylbenzylamine (aka a-phenethylamine, no simple benzylamine was handy). otto thougt to end up w/ tma2, but is sorry to see only the N-(a'-methyl)benzylamine.
could you give poor otto some hints?
otto
(Master Whacker)
02-18-02 05:28
No 271086
The N-methylbenzylamine group will split off in exactly the same manner as benzylamine via hydrogenation, however a good 10%Pd catalyst must be used. 5%Pd/C will only reduce the imine, it will not hydrogenolyze the benzyl group. I actually ran into this same problem once upon a time with 5% Pd/C, however 10%Pd/C worked perfectly. SWIM has also had excellent results with debenzylation reactions using Pearlmans catalyst-10%Pd(OH)2/C, but this is much more expensive than plain old 10%Pd/C. An interesting note about these reactions is that SWIM found out that even if a MASSIVE amount of 5% Pd/C was employed, reductive debenzylation will not occur, 10%Pd is a must! Also using at least 1atm H2 pressure should help a great deal.
(Newbee)
02-18-02 22:41
No 271410
thank you very much ritter,
the Pd/C otto used comes from a bottle saying "Pd/C" no percentage is given. have to check this out. also otto run out of hydrogen. have you any experience using HCOOH or zinc or cyclohexen as hydrogendonor?
otto read that hydrogenolysis is much easier at lower pH. could one possibly carry this out in acetic acid adding zinc for hydrogen-source?
excuse me for bothering.
otto
(Old P2P Cook)
02-18-02 22:52
No 271414
It seems that you would likely get better results by finding a way to get benzylamine to replace the alpha-methylbenzylamine that you are using.
(Distinctive Doe)
02-22-02 02:18
No 271694
(Rated as: excellent)
Here are some relevant threads
Post 465442 (Rhodium: "Hydroamination Route to Amphetamines", Chemistry Discourse)
Post 204565 (obituary: "Bu-Li catalyst", Chemistry Discourse)
Post 186651 (Dope_Amine: "Attempted Hydroamination of Safrole", Novel Discourse)
Post 42374 (CHEM_GUY: "The damn text.", Novel Discourse)
Below I reposted what I feel are the main two reactions of interest from this article.
Tetrahedron, Vol. 52, yr 2000, pages 5157-62
General procedure
The amine (2.5 mmol) was dissolved in 5 ml dry tetrahydrofuran in an Ace-pressure tube under an argon atmosphere. 20 mol% n-butyllithium (1.6 M n-BuLi solution in hexane) was added slowly at room temperature. The solution was stirred for 10 min and then allylbenzene (5 mmol) was added. The intensive coloured solutions were reacted for 20h at the defined temperature. After cooling to room temperature, the solution was quenched with 2 ml water, whereby a discolouration of the solution was observed. The products were isolated by acidification of the mixture with 5 ml of 1 M HCl followed by addition of 5 ml dichloromethane. The resulting aqueous phase was collected and the organic phase was extracted three times with 5 ml of 1 M HCl. The combined aqueous phases were neutralized with solid Na2CO3 and were extracted five times with 5 ml dichloromethane. The organic phases were washed with water and dried over MgSO4. After removal of the solvent in vacuo the products were isolated by column chromatography.
N-benzylamphetamine (5).
According to the general procedure benzylamine (2.5 mmol; 273 ul) and allylbenzene (5 mmol; 662 ul) were reacted in the presence of 20 mol% n-BuLi solution (0.5 mmol; 313 ul) at 50°C. The residue was purified by column chromatography (n-hexane/ethyl acetate=4:1) to afford 5 as a colourless oil. Yield: 65%(GC); 60%(isolated)
N-Butylamphetamine (7).
According to the general procedure n-butylamine (2.5 mmol; 247 ul) and allylbenzene (5 mmol; 662 ul) were reacted in the presence of 20 mol% n-BuLi solution (0.5 mmol; 313 ul) at 50°C. The residue was purified by column chromatography (n-hexane/ethyl acetate=1:2) to afford 7 as a colourless oil. Yield: 62%(GC); 54%(isolated)
Misc from article:
Tertiary (double alkylated) amines were produced in yields <1% in the reactions of allylbenzene with primary amines. This makes the method especially attractive for the selective synthesis of `secondary amphetamines' because typical nucleophilic substitution procedures with primary amines gave mixtures of secondary, tertiary and even quaternary amines. Furthermore, the reaction of benzylamine is noteworthy, since the benzyl group is easily removed by hydrogenation with H2/Pd/C to give 1-phenyl-2-aminopropane in 65% overall yield by GC analysis.
They give some conflicting data about the effect of temperature on the reaction. With piperazine the highest yeild occurs when the rxn is started at -78C and allowed to rise to room temperature. When aminating with morpholine the yeild is 44% at room temperature and 88% at 50C. They conclude that higher temperatures are better for yeild, in general, which in my opinion is totally unjustified based on the results they present. I have a suspicion that colder temperatures will increase the yeild(in THF) and would definately recommend that anyone attempting this try it cold also.(maybee even -78C)
They even state "Oligomers of beta-methylstyrene are obtained as side-products by an anionic polymerization reaction. The amount of oligomers increases at higher reaction temperature."
What would bee the best scheme for purifying the product from this reaction if it were performed on say Asarone to yeild N-benzylTMA-2. You'll have a bunch of unreacted Asarone, your N-benzylTMA-2, some polymer and possibly a bit of tertiary amine. Do you think an A/B followed by catalytic removal of the benzyl group and then a standard workup would bee sufficient?
Foxy just rambleing
This article might bee useful also
Synthesis of beta-Phenylethylamines from Styrene Derivatives
Tetrahedron Letters, Vol.39, 28 (1998) p.5073
(Master Whacker)
02-22-02 05:33
No 271776
terbium:
The alphamethylbenzylamine undergoes reductive amination then debenzylation in the exact same manner as plain old benzylamine. The only difference is that alphamethylbenzylamine has a chiral center so it can be used to produce pure enantiomers of the desired amine.
otto:
There is a CTH reduction typed up here in the archives which uses ammonium formate and Pd/C (a huge amount of 10%Pd/C) to produce very high yields of the primary amine from N-Benzylamines. This rxn should work fine for you, **HOWEVER** if the alphamethylbenzylamine was a pure enantiomer (Dextro or Levo, of course) your resulting TMA-2 is not going to consist of the desired racemic mix!!! This is important! Check that bottle of alphamethylbenzylamine to determine its chirality ASAP and let me know what it says.
(Stoni's sexual toy)
02-22-02 13:03
No 271982
Anionic polymerisations all have a unique 'ceiling temperature'. If you take a solution containing the non-terminated polymer anion and raise the temperature over the ceiling temperature depolymerisation sets in. And when you go below this ceiling temp the whole shebang starts to repolymerise.
But since BuLi is first reacted with an amine that shouldn't be issue, as long as the amide anion isn't a good starter for the polymerisation (don't think it is basic enough). Once you go over the ceiling temp any formed polymer will decompose again and probably react in the desired way. This might be an explanation for their conflicting temperature results, guess that ceiling temp depends on the counterion.
I'm not fat just horizontally disproportionate.
(Newbee)
02-22-02 21:50
No 272162
hi ritter,
otto used racemic a-methylbenzylamine. although it might be interesting to evaluate the isomers of tma-2. unfortunately otto was not able to get the benzyl cleaved off.
otto decided to do with plain ammonia and again went into a fuck-up. being confused about NaBH4-reduction and CTH he tried to hydrogenate a mixture:
300 mg tmp2p in 5 ml methanol, 500 mg ammonium acetate, 200 mg of his Pd/C.
after 4 hours under hydrogen (15% HCl into Zn in an extra vessel, connected by rubber-tube, at the outlet of hydrogenation vessel baloon) recovery of unchanged ketone.
must there be NO acid, only ammonia, otto is thinking?
otto
(Chief Bee)
10-18-03 19:17
No 465439
(Rated as: excellent)
Review: Base-Catalyzed Hydroamination of Olefins: An Environmentally Friendly Route to Amines
Jayasree Seayad, Annegret Tillack, Christian G. Hartung, Matthias Beller
Advanced Synthesis & Catalysis, 344(8), 795-813 (2002) (../rhodium/pdf /base-cat.hyd
DOI:10.1002/1615-4169(200209)344:8<795::A
Abstract
The base-catalyzed hydroamination of olefins offers a simple and elegant access to various primary, secondary, and tertiary amines. Particular focus is placed on developments in the area of hydroamination of non-activated olefins. Advantages and disadvantages of the methodology compared to other synthetic methods are presented. Special attention is paid to potential industrial applications of this chemistry.
(Chief Bee)
10-18-03 19:31
No 465442
(Rated as: excellent)
Here is the full article excerpted in Post 271694 (foxy2: "Re: here it is: direct amination of allylbenzene", Chemistry Discourse)
A Base-Catalyzed Domino-Isomerization–Hydroamination Reaction––A New Synthetic Route to Amphetamines
Christian G. Hartung, Claudia Breindl, Annegret Tillack and Matthias Beller
Tetrahedron 56(29), 5157-5162 (2000) (../rhodium/pdf /hydroaminati
DOI:10.1016/S0040-4020(00)00436-1
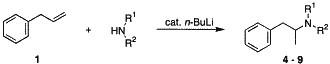
Abstract
An efficient synthesis of pharmaceutically interesting amphetamines by a base-catalyzed domino-isomerization–hydroamination reaction is presented. Starting from allylbenzene and various primary or secondary amines, the basic structural pattern of amphetamines is synthesized directly in yields of up to 91% in the presence of catalytic amounts of n-butyllithium.
(Chief Bee)
10-18-03 21:01
No 465452
(Rated as: good read)
Synthesis of beta-Phenylethylamines From Styrene Derivatives
Julio A. Seijas, M. Pilar Vázquez-Tato, César Entenza, M. MontserratMartínez, M. Gabriela Ónega and Susana Veiga
Tetrahedron Letters 39(28), 5073-5076 (1988) (../rhodium/pdf /hydroaminati
DOI:10.1016/S0040-4039(98)00907-1
Abstract
beta-Phenylethylamines are prepared from the styrene derivatives: 4,4-dimethyl-2-(2-vinylphenyl)-2-oxazoli