12-18-03 06:12
No 477537
My post stems from the fact that chloroacetic acid and sodium cyanide are not particularly easy to make or
friendly - i would like to find another way to synth that would use precursors that are easier to acquire even if the yields arent particularly great.
If anyone could help i would appreciate it - ive been poring over organic chemisty books for a good while now - the only detailed reference I've found is from vogel and i dont relish the thought of toying with sodium cyanide and chloroacetic acid - i found some more useful hints here - http://4.1911encyclopedia.org/M/MA/MALON
It still doesnt give enough details -- i have some permanganate and sulfuric around -- i wonder if they could perform the same oxidation or if they would spoil malic acid(easy to acquire) - or maybe i could oxidize with a copper catalyst - would love any ideas/leads - thanks
(Stranger)
12-19-03 00:10
No 477705
found an interesting patent here -- might try it out
http://l2.espacenet.com/espacenet/viewer
anyone have a good test for determination of malonic acid?
(Hive Bee)
12-20-03 17:58
No 478005
what about sodium succinate with strong base (eg. sodium tert-butoxide, or calcium carbide) for alpha dehydrogenation?
if that wouldn't work a synth for an alpha-halo succinic acid or ester followed by a treatment with strongbase probobly would.
(Stranger)
12-20-03 18:11
No 478007
hmm would that work? i looked again at my patent and found that calcium cyanoacetate is pretty hard to get hold of as well (i couldnt even find a MSDS for it) - so no go there - will keep searching
(Hive Bee)
12-20-03 21:49
No 478037
wait, i was getting confused with malic acid.
you might find this link helpful
http://4.1911encyclopedia.org/M/MA/MALON
it mentiones it can be extracted from the sugar beat, id also do some research into some other bio-sources and bio-synthesis, as it seems to be present in alot of plants.
also malic acid can be purchased otc as a vitamin, the link above mentions that it can oxidized to malonic acid, im not sure if it is best done chemically or biologically (for instance with yeast or something)
(Chief Bee)
12-20-03 22:55
No 478056
if there is a way to make 2-halo-succinic acid
Yup, it's called the Hell-Volhard-Zelinsky reaction (http://themerckindex.cambridgesoft.com/
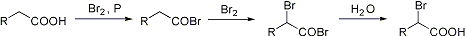
The Hive - Clandestine Chemists Without Borders
(Stranger)
12-21-03 02:57
No 478091
Yes -- oxidation of malic acid was my first idea -- the page http://77.1911encyclopedia.org/M/MA/MALI
says that potassium bichromate can be used but i cant find a reference for this reaction anywhere - temperatures/amounts - I have even found some books saying contrary things about oxidation of malic acid -- was wondering if permanganate/sulfuric could be used instead of the chromate ion -- anyone know how determine an organic acid besides spectro or mass calc? I'm pretty sure malonic is much more acidic than malic.
How does a halogenation reaction fit into this Rhodium? please explain.
(Chief Bee)
12-21-03 03:51
No 478104
I just answered the question in Post 478005 (stratosphere: "what about sodium succinate with strong base...", Chemistry Discourse)
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
12-22-03 21:33
No 478497
my thought on 2-halo-succinic was in regards to malic so isn't entirly relevant here.
but for the case of 2-halo-succinic acid is the Hell-Volhard-Zelinsky going to give you any 2,3-dihalo-succinic, or is the 2-halo suffeciently deactivating to keep the 3 halogenation from occuring?