12-15-03 06:17
No 476990
has anyone once EVER tried to see if lithium will dissolve in anhydrous diethylamine? it would be SO SIMPLE to test and it would change EVERYTHING if it work - even if you need a cosolvent like tertbutanol - isn't this worth a TRY?
"Decay is inherent in all compounded thing. Strive unceasingly."
(Stoni's sexual toy)
12-15-03 06:29
No 476993
Has DEA suddenly become available OTC for like real cheap or what?
I'm not fat just horizontally disproportionate.
http://www.antiwar.com
(Stranger)
12-16-03 05:07
No 477169
how cheap is andydrous ammonia? and EDTA IS dirt cheap and every where. besides, EDA is safe and easy to work with, pure NH3 is corrosive, deadly and utterly watched - i'm just saying, wouldn't it be worth a try?
and DEA diethylamine, is not the same as EDA, ethylenediamine - besides the instant panic the initials DEA cause....
and yes, i screwed up in the original post and called it diethylamine - i meant ethylenediamine, sorry
"Decay is inherent in all compounded thing. Strive unceasingly."
(Stoni's sexual toy)
12-16-03 06:16
No 477172
> how cheap is andydrous ammonia?
Very cheap.
> EDTA IS dirt cheap and every where. besides, EDA is safe and easy to work with,
EDTA is not EDA. And DEA is yet another chemical.
> pure NH3 is corrosive,
Just like the other amines.
> deadly
Not much more deadly than the other amines.
> and utterly watched
Not nearly as watched as DEA.
I'm not fat just horizontally disproportionate.
http://www.antiwar.com
(Moderator)
12-16-03 06:26
No 477174
(Rated as: excellent)
It doesn't work the way that you think, read these for the reason why
Synthesis 391-415 (1972)

Organic Reactions 42, 1-47; 320-334 (1992)

J. Am. Chem. Soc. 77, 3230 (1955)

J. Org. Chem. 48, 2796(1983)

J. Am. Chem Soc. 74, 5699(1952)

Tetrahedron Letters no. 16, 1-3 (1960)

JCS Perkin I. 475-7 (1975)

Synlett 344-7 (1993)

Chemistry is our Covalent Bond
(Stranger)
12-16-03 06:46
No 477177
anhydrous ammonia is cheap - but very well guarded. and what does DEA have to do with anything? EDTA is ethylenediamine tetracidic acid - can this not be reacted with a base to make EDA? the "metal ammonia solutions" book very clearly states that lithium will dissolve in an diamine. sorry, though, i'm on dial up and it will take me a while to read over those refs.
"Decay is inherent in all compounded thing. Strive unceasingly."
(Stranger)
12-16-03 06:55
No 477181
Dimethyl Ethylene gycol ether. Na/K - very soluble K - moderate Na, Li, Ca - None Ethyl Methyl Ethylene Glycol ether Na/K - slight Dimethyl Diethylene glycol ether Na/K - very soluble Diethyl Diethylene Glycol ether Na/K - slightly Ethyl Methyl Diethylene Glycol ether Na/K - Moderate Methyl n-propyl Diethylene Glycol ether Na/K - slightly (a) n-Butyl Methyl Diethylene Gycol ether Na/K - very slightly (a) TetraHydroFuran Na/K - slightly 1-Methoxymethyltetrahydrofuran Na/K - Very soluble 1-ethoxymethyltetrahydrofuran Na/K - moderate (b) 2-Methyltetrahydrofuran Na/K - very slightly (a) Dioxan Na/K - None Cyclic tetramer of propylene oxide Na/K - Very soluble 1: 2-Dimethoxypropane Na/K - very slightly (a) Triethylene glycol dimethyl ether Na/K - very soluble Tetraethylene glycol dimethyl ether Na/K - very soluble Ethylenediamine very soluble Na/K - very Methoxyethylamine Na/K - very soluble
(only on proplonged cooling to 193 K)
( slightly at room temp; moderate on cooling to 193 K)
This article goes on to state:
Lithium - doesn't dissolve in dimethylamine but is quite soluble in ethylamine
and BTW, i did read the ENTIRE metal-ammonia solution book
ps - i'm having not luck what so ever trying to read those refs what format are they in? i mean, i can download them, but them my computer has NO idea how to deal with them
"Decay is inherent in all compounded things. Strive unceasingly."
(Moderator)
12-16-03 07:15
No 477185
If you'd looked at the top of:
../rhodium /index.h
You would have found this link:
http://www.lizardtech.com/download
Chemistry is our Covalent Bond
(Stoni's sexual toy)
12-16-03 08:08
No 477186
> EDTA is ethylenediamine tetracidic acid - can this not be
> reacted with a base to make EDA?
No. EDTA is NOT the salt of one molecule of ethylenediamine and 4 molecules of acetic acid! You cannot remove the acetic acid part with base, since it isn't a salt.
> and what does DEA have to do with anything?
Well, you mentioned it in the thread title: "lithium and diethylamine"!
Ethylenediamine and diethylamine are two different compounds.
I'm not fat just horizontally disproportionate.
http://www.antiwar.com
(Chief Bee)
12-16-03 08:45
No 477189
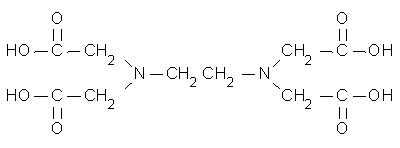
EDTA - Ethylenediaminetetraacetic Acid
The Hive - Clandestine Chemists Without Borders
12-16-03 18:59
(Rated as: Insignificant, Stimulants topic, UTFSE!!)