(Stranger)
09-22-00 01:33
No 54080
(Rated as: excellent)
High-yielding synthesis of MDA from MDP2P
(For informational purposes only!)
This route to MDA isn't anything new. In fact it has been around since 1955.
The general overview of the reaction is; 1) Formation of the oxime and 2) reduction of the oxime to the amine. The fist step is easily accomplished by reacting the parent ketone with hydroxylamine hydrochloride. Old geezer Shulgin did this with pyridine [1] in a 51% yield. But it can be done with considerably better yields employing plain sodium acetate and methanol [2].
I heard the following through my friends appartment wall...
Step 1. Formation of the oxime.
5.4g sodiumacetate trihydrate and 4ml water was combined in a 50ml roundbottomed flask. The mixture was heated gently with stirring until the acetate was in solution. 20 ml MeOH was added followed with 4.5g MDP2P. To this there was added 2.3g hydroxylamine hydrochloride and the mixture was refluxed with stirring for 1.5h. After this period 10ml water was added and the heating source was removed and the mixture was allowed to cool in a waterbath with the stirring continued. After returning to room temp the flask was put in the freezer for an hour or so. The white crystalline material was filtered and washed with 50ml water (the filtrate may turn cloudy as small amounts of product crystallizes). The product was dried over magnesiumperchlorate. Final weight was 4.5g (92%). Mp: 84-85°C. Litt:84-87°C (H2O-EtOH)
The oxime can also be made in equally good yields with sodiumcarbonate as the base in 60% EtOH [3].
The reduction is done with sodium metal in EtOH[2,4]:
Step 2. Reduction of the oxime.
3.86g (0.02 mole) oxime was dissolved in 50 ml dry ethanol (fresh 99.5% is OK, otherwise look in Vogel for a procedure for making anhydrous EtOH with Mg and I2) in a two-necked 250ml roundbottomed flask equipped with a condenser, a cork and a stirring magnet. The reactionmixture was heated to reflux, the heat was turned off and 5g elemental sodium was added in such rate that a steady reflux was maintained (make 20-30 pieces and store under hexane). The first additions is conviently kept small. (NOTE: Hydrogen evolution). At the end the reaction was slower so the heating mantle was turned back on to speed things up.
The waterwhite, clear postrxn mixture was then slowly treated with 16g H2SO4 in 200ml cold water.
The EtOH was removed under vacuum, and the resulting waterphase was washed twice with DCM.
The aq. phase was made basic by addition of 25% NaOH. The freebase was extracted with 3x30ml DCM. The pooled organic extracts was evaporated under vacuum to constant weight leaving a pale colored residue (3.36g, 94%).
The freebase was dissolved in 20ml IPA, neutralized with 1.6ml HCl and percipitated by the addition of 40 ml Et2O. The crystals was filtered and washed with a small amount of Et2O.
Yield: 3.4g of white MDA-HCl. Mp 187.5-188.5 C. (Litt: 187-188°C)
This reduction can also be done in n-BuOH according to [5], and with quite a few NaBH4 reduction systems (NiCl2 [6], TiCl4[7], MoO3[8], Co-PC[9], amberlyst-15+LiCl[10], and even with plain NaBH4 in EtOH according to [11]). Adding NaOH to a suspension of Ni-Al is also said to reduce oximes[12] as is SnCl2-Sn-HCl[13].
Different synthesis of oximes starting with nitroalkenes should be looked into, as this could be a good alternative to the existing methods for reducing nitroalkenes.
SnCl2 reduces nitroalkenes to oximes under both acidic and basic conditions[14, 15, 16). Zn(BH4)2 in 1,2-DiMeO-ethane does reduce 3,4-MD-phenyl-2-nitropropene to the oxime in good yields [17]. Another promising route is the use of Pb in DMF [18]. This seems to reduce most nitroalkenes to the corresponding oximes.
Refs:
1. J. Pharm. Sci. 69; 2; 1980; 192-195
2. CA 1955;10595
3. Chem. Ber. 114;12;1981;3813-3830
4. J. Chem. Soc. Perkin. Trans. 2;2;1997;249-256
5. JACS 56; 1934; 487
6. Chem. Pharm. Bull. 36;1988;1529-1533
7. Synthesis 9;1980;695-697
8. Chem. Ber. 117;2;1984;856-858
9. Angew. Chem. 93;5;1981;477-479
10. Syn. Lett. 4;1999;409-410
11. J. Chem. Soc. Perkin. Trans. 2; 1980; pp83-86 (very sparse information)
12. Aust. J. Chem 34; 1;1981;45-56
13. JACS 67;1945;496
14. Tetr. Lett. 26;49;1985;6013-6014
15. Synth. Commun. 18;7;1988;693-698
16. Heterocycles 24;9;1986; 2581-2586
17. Tetrahedron 48;25;1992; 5317-5322
18. Tetrahedron 48;21;1992; 3313-26 among others
(Hive Bee)
09-25-00 14:05
No 54821
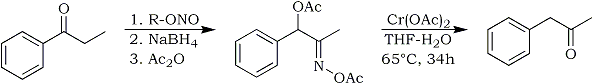
Removal of Oxime group by conversion to the acetate ester, followed by chromous ion reduction.
E.J. Corey and J. E. Richman, J. Am. Chem. Soc.
92, 5276 (1970)
This could be an article of interest, i have not looked it up yet but i thought i'd throw it out to the rest of you.
If anyone wants to find it and comment that would be appreciated.
Foxy Out
(Chief Bee)
09-14-04 21:59
No 531390
(Rated as: good read)
As mentioned in Post 54821 (foxy2: "Re: High-yielding synthesis of MDA from MDP2P", Methods Discourse)
Reaction of oxime O-acetates with chromous acetate.
Method for the conversion of ketoximes to ketones under mild conditions
Elias J. Corey, Jack E. Richman;
J. Am. Chem. Soc., 92, 5276-5277 (1970) (../rhodium/pdf /chromous.ace
The Hive - Clandestine Chemists Without Borders
(Stranger)
09-14-04 23:51
No 531410
Yes this looks like a good reaction. Beta-keto-phenylalkanes may have interesting applications in novel synthetic protocols. I had some ideas a few months ago but abandoned them for lack of a good route. Perhaps this could help fill this void?