(Hive Addict)
03-31-03 07:33
No 422757
(Rated as: excellent)
New reagents 31a,b: aluminium iodide, a highly regioselective ether-cleaving reagent with novel cleavage pattern
MV Bhatt, JR Babu
Tetrahedron Letters 25(32) (1984) 3497-3500
Abstract - AlI3 is an easily accessible and versatile ether-cleaving reagent.
Article - Boron and silicon halides have been widely used for the cleavage of dialkyl and aryl alkyl ethers. 1a,b,2,3,4 Although AlCl3 5 has been employed for the cleavage of certain types of ethers, its usefulness for ether cleavage is rather limited. Brief reports on the ether-cleaving property of AlBr3 and AlI3 have not been followed up to explore the full synthetic potential of these reagents.6,7,8
The Lewis acid strenght of aluminium halides increase in the order 9: AlCl3 < AlBr3 < AlI3. One could expect AlI3 to be a highly reactive reagent.
Inverse reactivity pattern - In CH3CN, AlI3 cleaves aromatic aliphatic ethers much faster than dialkyl ethers. For example, anisole is cleaved in 12 hr at 80°C, whereas cyclohexyl methyl ether requires 52 hr under the same conditions. This is further illustrated in the case of 1-methoxy-2-phenoxy ethane. We find AlI3 alone gives phenol, whereas other reagents give 2-phenoxy ethanol (see Table I). This behaviour of AlI3 is in contrast to the normal reactivity pattern of silicon and boron reagents (Scheme I).
Scheme I
| |
Ph-|-O---CH2-CH2---O-|-Me
| |
AlI3 -> | | <- BCl3, BBr3,
| | Cl3SiI, Me3SiI
Table I - Cleavage of PhOCH2CH2OMe with various reagents
+-------+----------------+-------------+-------- --+----- -------- -------- -------- --+
|REAGENT| MOLE RATIO | TIME (HR)/ | SOLVENT | PRODUCTS ISOLATED YIELD (%) |
| | SUBSTR:REAGENT | TEMP (°C) | | PhOH | PhOCH2CH2OH |SUBSTR |
+-------+----------------+-------------+-------- --+----- ----+--- -------- --+----- --+
AlI3 1:1 4/82 MeCN 75.07 - -
BCl3 3:1 15/65 CHCl3 traces 47 25.4
BCl3 1:1 11/65 CHCl3 33 58.7 -
BBr3 3:1 22/25 CH2Cl2 traces 44.3 19.04
Me3SiCl 1:1 12/25 MeCN traces 55.1 33.5
/NaI
SiCl4 1:1 16/25 MeCN/ traces 75 6
/NaI CH2Cl2
(1:1)
+-------+----------------+-------------+-------- --+----- ----+--- -------- --+----- --+
Novel solvent effects - Another noteworthy feature is that the rates of cleavage of certain ethers are reversed in CS2 and in CH3CN. 1,3-benzodioxole (0.5 hr) and o-dimethoxybenzene (0.5 hr) are cleaved faster than anisole (12 hr) in CH3CN whereas the reverse is observed in CS2 (see Table II).
In CS2 and in C6H6 secondary alkyl groups, after they are converted to the corresponding iodides during ether cleavage undergo isomerization to give a mixture of products. This does not take place in CH3CN medium in which even labile ether like allyl phenyl ether gives phenol and allyl iodide. No ring alkylation products could be detected (see Table II, entry 3)
Table II - Cleavage of ethers with AlI3
ENTRY SUBSTRATE SUBSTRATE: PRODUCT SOLVENT TIME OF YIELD(%)
REAGENT SYSTEM REFLUX (HR)
RATIO
------------------------------------------------ -------- -------- -------- -------- --------
1. anisole 1:1 phenol A 12 94
1:1 phenol B 1 90.3
1:1 phenol C 1.5 90.4
2. p-dimethoxy- 1:1 4-MeO-phenol C 3 74.2
benzene 2:1 hydroquinone C 4 85
3. allyl phenyl 1:1 o-allylphenol C d 26
ether phenol 4.5
1:1 phenol A 5 89
4. 1,3-benzo- 1:1 catechol A 0.5 70.5
dioxole 2:1 catechol B 5.5 68.0
2:1 catechol C 7 80
7. o-dimethoxy- 3:1 catechol C 19 84
benzene guaiacol 2
2:1 catechol B 7.5 91
1:1 catechol A 0.75 80
9. tetrahydro- 1:1 4-iodobutanol A 5 70
furan 1,4-diiodobutane 5.8
------------------------------------------------ -------- -------- -------- -------- --------
Solvent system: A = CH3CN, B = benzene, C = CS2.
d = 3.5 hours at ca -70°C
Preparation of AlI3 - Dry aluminium foil (250 mg, 9.3 mmol) and iodine (1.9 g, 15 mmol) were refluxed in dry CS2 (10 mL) or dry CH3CN (8 mL) till the iodine colour disappeared (ca 3 hours).
Cleavage of 1,3-benzodioxole in CS2 - To a freshly prepared solution of AlI3 (10 mmol) in CS2, 1,2-benzodioxole (610 mg, 5 mmol) in CS2 (2 mL) was added and refluxed till there was no more starting material (7 hours, TLC). The cooled reaction mixture was decomposed with ice, extracted with ether and washed with thiosulphate solution. The thiosulphate solution was extracted once again with ether and the combined ether extract was dried over anhydrous MgSO4. The solvent was removed and the product was chromatographed (TLC silica gel, 3:1 hexane:EtOAc) to obtain catechol, 440 mg (80%), mp 106°C (Lit 10 mp 104.8-105.8°C).
Cleavage of allyl phenyl ether in CH3CN - To a freshly prepared solution of AlI3 (5 mmol) in CH3CN, allyl phenyl ether (670 mg, 5 mmol) in CH3CN was added and the concentration of the solution was adjusted to ca 1 M with respect to both reagent and reactant. The reaction mixture was refluxed till there was no more starting material (5 hours, TLC), cooled and poured into water. The mixture was extracted with ether and the aqueous extract was washed with 5% NaOH solution. After acidification of the alkaline aqueous solution, it was extracted into ether, dried over anhydrous MgSO4 and the solvent was removed. The crude product after short path distillation yielded phenol; 418 mg (89%).
References
1a. MV Bhatt, J Organomet Chem 156, 221 (1978)
1b. MV Bhatt e.a., Synthesis 1048 (1982)
2. for a recent review, see "Cleavage of ethers". MV Bhatt e.a., Synthesis 249-282 (1983)
3. ME Jung e.a., JOC 42, 3761 (1977); Synthesis 588 (1978); Tetrahedron Lett 3657 (1978)
4. GA Olah e.a., JOC 44, 1247 (1979); TL Ho e.a., Angew Chem 88, 847 (1976); Angew Chem Int Edn Eng 15, 774 (1976)
5. C Hartmann e.a., Ber 25, 3531 (1892)
6. P Pfiffer e.a., J prakt Chem NF 147, 293 (1937)
7. E Mincione, Ric Sci 39, 424 (1969)
8. S Cabiddu e.a. Ann di Chim 62, 505 (1972)
9. DPN Satchell e.a., Chem Rev 69, 251 (1969); JKM Divitt e.a., Spectrochim Acta 30, 1021 (1974)
10. JJ Lander e.a., JACS 67, 322 (1945)
Acknowledgements - The authors wish to express their appreciation to CSIR for funding this project.
The faster you run, the quicker you die.
(Newbee)
07-03-03 11:08
No 444270
(Rated as: excellent)
Synthetic Communications, 1999, 29(6), 973-979 (http://www.angelfire.lycos.com/scifi2/l
Dealkylation of alkyl and aryl ethers with AlCl3-NaI in the absence of solvent
[...]
General procedure
A mixture of alkyl or aryl ether (1 equivalent), AlCl3 (2 equivalent) and NaI(2 equivalent) was thoroughly ground in an agate mortor for a few minutes. The softened mass was ground by heating (70-80 °C) for 1-3 hrs. The reaction mixture was diluted with the aqueous solution of Na2S2O3 (5%) and extracted with ether. The ether extract was dried over MgSO4. The solvent was removed in vacuum to give the product.
Methylsalicylate
A mixture of methyl 2-methoxybenzoate (1.66 g, 10 mmol), anhydrous aluminium chloride (2.66 g, 20 mmol) and sodium iodide (2.89, 20 mmol) was thoroughly ground in an agate mortor for a few minutes. The reaction mixture was ground by heating (70-80 °C) for 2hr. The reaction was diluted with 20 ml of aqeous Na2S2O3 (5%) and extracted with 2x20 ml Et2O. After drying the organic layer over anhydrous MgSO4 the solvent was removed on a rotary evaporator, providing 1.45 g 95% of methyl salicylate.
[...]
Table 1. Dealkylation of alkyl or aryl ethers to corresponding alcohol or phenol
| Entry | Substrate | Time (hrs) | AlCl3:NaI | Temp. (°C) | Products | Yield bases on TLC( %)a |
| 1 | Di-n-butyl ether, (n-C4H9)2O | 15 min | 2:2:1 | 70-80 | n-Butanol, n-C4H9OH | 100 (95) |
| 2 | Palmityl methyl ether, n-C16H33OCH3 | 15 min | 2:2:1 | 70-80 | n-Palmitol, n-C16H33OH | 100 (97) |
| 3 | Anisole, methoxybenzene | 15 min | 2:2:1 | 70-80 | Phenol | 100 (98) |
| 4 | 2-methoxytoluene | 3.5 | 2:2:1 | 70-80 | o-Cresol | 100 (97) |
| 5 | 4-methoxytoluene | 2 | 2:2:1 | 70-80 | p-Cresol | 100 (97) |
| 6 | Veratrol, 1,2-dimethoxybenzene | 8 | 2:2:1 | 70-80 | 2-Methoxy phenol | - (65) |
| 7 | Veratrol, 1,2-dimethoxybenzene | 24 | 2:2:1 | 70-80 | pyrocatechol, 1,2-dihydroxy benzene | 100 (95) |
| 8 | Resorcinol dimethylether, 1,3-dimethoxybenzene | 4 | 2:2:1 | 70-80 | 3-Methoxy phenol | - (70) |
| 9 | Resorcinol dimethylether, 1,3-dimethoxybenzene | 24 | 2:2:1 | 70-80 | Resorcinol, 1,2-dihydroxy benzene | 100 (95) |
| 10 | Hydroquinone dimethylether, 1,4-dimethoxybenzene | 2 | 2:2:1 | 70-80 | 4-Methoxy phenol | 100 (96) |
| 11 | Hydroquinone dimethylether, 1,4-dimethoxybenzene | 2 weeks | 2:2:1 | 70-80 | Hydroquinone, 1,4-dihydroxy benzene | 100 (95) |
| 12 | 2-nitromethoxybenzene | 24 | 2:2:1 | 70-80 | no reaction | - |
| 13 | Methyl 2-methoxybenzoate, 2-Methoxy benzoic acid methyl ester | 2 | 2:2:1 | 70-80 | methyl salicylate | 100 (95) |
| 14 | 3-Bromo anisole | 3 | 2:2:1 | 70-80 | 3-Bromo phenol | 100 (96) |
| 15 | Tetrahydrofuran, THF | 45 min | 2:2:1 | 70-80 | 4-iodo butanol | 100 (90) |
| 16 | C28H46OCH3 | 4 | 2:2:1 | 70-80 | no reaction | - |
| 17 | (methoxymethyl)benzene, benzyl methyl ether | 1 | 2:2:1 | 70-80 | (iodomethyl)benzene, benzyl iodide | 100 (60) |
| 18 | 1-Methoxy naphthalene | 3 | 2:2:1 | 70-80 | - | - |
| 19 | 2-Methoxy naphthalene | 3 | 2:2:1 | 70-80 | 2-naphthol | - (20) |
| 20 | (allyloxy)benzene, phenyl allyl ether | 1 | 2:2:1 | 70-80 | mixture of products | - |
a Number in the parantheses are isolated yields
[...]
A practical disadvantage of this method could be liberation of small amount of iodine that would harm a sensitive functional group (entry 20).
[...]
Lego's voice: Entry 15 might bee of special interest for the synthesis of DMT via phenylhydrazine as 4-iodobutanol can bee converted to 4-(N,N-dimethylamino)butanal dimethyl acetal in two steps with OTC chemicals.
The candle that burns twice as bright burns half as long
(Newbee)
07-03-03 14:54
No 444300
(Rated as: excellent)
Dealkylation of activated alkyl aryl ethers using lithium chloride in dimethylformamide
Synthesis, 1989, 4, 287-289 (http://www.angelfire.lycos.com/scifi2/l
Alkyl aryl ethers having electron-withdrawing substituents in the ortho or para positions are easily cleaved with lithium chloride in dimethylformamide.
[...]
Table 1. Cleavage of alkyl aryl ethers 1a-q with LiCl in DMF
| Ethera | R | X1 | X2 | Reaction time (h) | Yieldb (%) | Phenol | mp (°C) or bp (°C)/Torr - found | mp (°C) or bp (°C)/Torr - reported |
| 1a | Me | 2-NO2 | H | 6 | 98 (92)17 | 2a | 44 | 4518 |
| 1b | Me | 3-NO2 | H | 6 | 50 | 2b | 95 | 9618 |
| 1c | Me | 4-NO2 | H | 24 | 98 (92)19 | 2c | 115 | 11418 |
| 1d | Et | 2-NO2 | H | 22 | 90 | 2d | 44 | 4518 |
| 1e | i-Pr | 2-NO2 | H | 24 | 35 | 2e | 44 | 4518 |
| 1f | PhCH2 | 2-NO2 | H | 22 | 98d | 2f | 44 | 4518 |
| 1g | Et | 4-NO2 | H | 24 | 10 | 2g | 112 | 11418 |
| 1h | Me | 2-NO2 | 4-Br | 4 | 95a | 2h | 90 | 8923 |
| 1i | Me | 2-Br | H | 72 | 67f (81)20 | 2i | 90/14 | 194-195/76018 |
| 1j | Me | 2-Cl | H | 72 | 55 (80)17 | 2j | 63/14 | 175-176/76018 |
| 1k | Me | 2-CHO | H | 22 | 70 (48)21 | 2k | 83/13 | 196.5/76018 |
| 1l | Et | 2-CHO | H | 22 | 25 | 2k | 83/13 | 196.5/76018 |
| 1m | i-Pr | 2-CHO | H | 22 | 5 | 2k | 83/13 | 196.5/76018 |
| 1n | Me | 2-CO2Me | H | 22 | 90g (89)14 | 2n | 161 | 15918 |
| 1o | CH2CO2Me | 2-Me | H | 22 | 5h | 2o | 188/760 | 191.5/76018 |
a Except for 1h16, ethers 1 were either commercially available (Aldrich Chemie) or easily prepared from the corresponding phenols
b Yield of isolated product. In brackets, reported yields.
c Identified by NMR spectrometry and by comparison of the physical properties with those of authentic samples
d Benzyl chloride yield was isolated in almost quantitative from the material insoluble in aqueous sodium hydroxide
e Traces of 4-chloro-2-nitro-phenol were detected
f 2-Chlorophenol was detected in 6% yield
g Salicylic acid was the only isolated product
h 2-Methylphenoxyacetic acid was recovered in 90% yield.
Table 2. Cleavage of Di- and Trimethoxybenzenes 1p-r and of Dimethoxybenzaldheydes 1s-u with LiCl in DMF
| Ethera | R | X1 | X2 | Reaction time (h) | Yieldb (%) | Relative Content (%) of Isomerc | Phenol | X1 | X2 | mp (°C) or bp (°C) - found | mp (°C) or bp (°C) - reported |
| 1p | Me | 2-OMe | H | 25 | 8 | - (90)22 | 2p | 2-OMe | H | 92/14 | 106.5/2418 |
| 1q | Me | 3-OMe | H | 25 | 7 | - (89)22 | 2q | 3-OMe | H | 124/10 | 244.3/76018 |
| 1r | Me | 2-OMe | 3-OMe | 48 | 60 (60)22 | 75d (30)22 | 2r | 2-OMe | 6-OMe | 55 | 55-5618 |
| 12 (30)22 | 2r' | 2-OMe | 3-OMe | 121/12 | 124-125/1718 | ||||||
| 1s | Me | 2-CHO | 6-OMe | 22 | 98 | - | 2s | 2-CHO | 6-OMe | 43 | 42-4324 |
| 1u | Me | 2-CHO | 5-OMe | 22 | 70 | 60 | 2t | 2-CHO | 5-OMe | 40 | 41-4225 |
| 1u | 40 | 2t' | 3-OMe | 4-OMe | 152 | 15326 | |||||
| 1u | Me | 4-CHO | 2-OMe | 22 | 61 | 44e | 2u | 2-OMe | 4-CHO | 80 | 81-8218 |
| 1u | 42 | 2u' | 2-OMe | 5-CHO | 115 | 11618 |
a All ethers and phenols except for 2t'26 were purchases from Aldrich-Chemie, Germany
b Yield of isoalted product or mixture of isomers. In brackets: reported yield
c Determined by GLG; in brackets: reported percentages. The products were also seperated by column chromatography (silica gel, light petroleum/Et2O, 3:1) and compared with authentic samples
d 3-Methoxy-1,2-dihydroxybenzene was present in 13% yield
e 3,4,-Dihydroxybenzaldehyde was detected in 14% yield.
2-Hydroxy-3-methoxybenzaldehyde (2s); Typical procedure
2,3-Dimethoxybenzaldehyde (1s; 1.0 g, 6 mmol) and LiCl (0.76 g, 18 mmol) are heated in DMF (10 ml), the reaction being monitored by GLC (2m SE-30 packed column). When the starting material has disappeared (22 h), 10% aqueous NaOH (30 ml) is added, the solution is washed with Et2O (2 x 25 ml), then acidified with 10% aqueous HCl (50 ml), and extracted with Et2O (2 x 25 ml). The organic phase is washed with brine (30 ml), dried (Na2SO4, and concentrated in a rotavapor; yield: 0.88 (98%); mp 42°C; mixture mp with a commercial sample (Aldrich): 42°C.
References
14. Synthesis, 1980, 638
16. Rec. Trav. Chim. Pays-Bas, 1937, 56, 541
17. Bull. Soc. Chim. Fr., 1970, 3647
18. Handbook of Chemistry and Physics, 1958
19. Bull. Soc. Chim. Fr., 1974, 2631
20. J. Am. Chem. Soc., 1942, 64, 1128
21. Synth. Commun., 1979, 341
22. Tetrahedron, 1982, 38, 3687
23. Rec. Trav. Chim. Pays-Bas, 1943, 62, 12
24. J. Chem. Soc., 1930, 2279
25. J. Chem. Soc., 1932, 1439
26. J. Chem. Soc., 1931, 84.
See also Patent EP1136481 (page 4) (already mentioned by Post 291887 (foxy2: "Eugenol demethylation", Novel Discourse)) for the demethylaton of eugenol to 4-allycatechol in 50% yield.
A solution of 354 ml (2.30 mmol) of eugenol and 292 g (6.89 mol) of lithium chloride in 3.7 l of N,N-dimethylformamide was refluxed for a total of 44 hours (h), and, after 4h, 18 h and then 7 h, a further 292 g (6.89 mol) of lithium chloride were added each time. After cooling, 2 l of toluene were added and the resultant precipitate was filtered off with suction and extracted with toluene. The organic extracts were combined and concentrated on a rotary evaporator. After flash chromatography (ether/pentane, 1:1 Rf - 0.37) on silical gel, 173 g (50%) of 4-allylcatechol were obtained.
The candle that burns twice as bright burns half as long
(Hive Bee)
07-09-03 14:04
No 445940
(Rated as: excellent)
Eur. J. Org. Chem. 2003, 1681-1686 (http://www.angelfire.lycos.com/scifi2/l
DOI:10.1002/ejoc.200210666
We have discovered serendipitously that chloroaluminate ionic liquids can cleave aromatic methyl ethers under surprisingly mild conditions. Three ionic liquids, viz. [TMAH]-[Al2Cl7], [BMIM][Al2Cl7], and [EMIM][Al2Cl6I], and aluminum chloride were compared in the selective demethylation of 4,5-dimethoxyindanone at the 4-methoxy-function. The ionic liquids exhibited a remarkably high selectivity (96:4) in comparison with aluminum chloride (70:30). In addition, the reaction time was drastically shortened when the ionic liquids were used. Interestingly, the three ionic liquids displayed the same reactivity in the demethylation of 4,5-dimethoxyindanone. Considering the lower cost and the bulk availability of the precursors of [TMAH][Al2Cl7], we conclude that this is the most attractive ionic liquid from an industrial point of view. To make the large-scale application of [TMAH][Al2Cl7] feasible, we have developed a safe upscalable method for its preparation. Furthermore, the scope of ether cleavage by the ionic liquid reagent [TMAH][Al2Cl7] was investigated and it was found that aromatic methyl-, allyl-, and benzyl-ether cleavage is applicable to a variety of heterocyclic compounds.
Table 2. Cleavage of aromatic methyl, allyl and benzyl ethers by [TMAH][Al2Cl7] (I.L.)a
| Entry | Substrate | Conversion (%)b, I.L. | Conversion (%)b, AlCl3 |
| 1 | Molecule: 1 ("c12cccc(c2CCCC1=O)OC") |
99 | 0 |
| 2 | Molecule: 2 ("c12ccc(cc2CCCC1=O)OC") |
99 | 63 |
| 3 | Molecule: 3 ("c12cc(ccc2CCCC1=O)OC") |
99 | 9 |
| 4 | Molecule: 4 ("c12ccc(c(c2CCCC1=O)[17O]C)OC") |
97 (99:1 | 97 (99:1) |
| 5 | Molecule: 5 (" c12cc(c(cc2CCCC1=O)OC)[17O]C") |
99 (99:1) | 97 (99:1) |
| 6 | Molecule: 6 ("c1cc2C(CCc2c(c1)OC)=O") |
99 | 0 |
| 7 | Molecule: 7 ("c1cc2C(CCc2cc1OC)=O") |
99 | 56 |
| 8 | Molecule: 8 ("c1(cc2C(CCc2cc1)=O)OC") |
99 | 0 |
| 9 | Molecule: 9 ("c1cc2C(CCc2c(c1OC)[17O]C)=O") |
99 (96:4) | 89 (70:30) |
| 10 | Molecule: 10 ("c1(cc2C(CCc2cc1OC)=O)[17O]C") |
99 (50:50) | 91 (90:10) |
| 11 | Molecule: 11 ("c1c(cc2c(c1)nccc2)OC") |
99 | 36 |
| 12 | Molecule: 12 ("c1c(cc2c(c1)cncc2)OC") |
99 | 0 |
| 13 | Molecule: 13 ("c1c(cc2c(c1)c[n+](cc2)[O-])OC") |
99 | 0 |
| 14 | Molecule: 14 ("c21cc(ccc1ncc2)OC") |
99 | 0 |
| 15 | Molecule: 15 ("c21cc(ccc1ncc2)OCc3ccccc3") |
99 | 0 |
| 16 | Molecule: 16 ("c21cc(ccc1nc(c2)C)OC") |
90 | 0 |
| 17 | Molecule: 17 ("c1(cnccc1)OC") |
99 | 99 |
| 18 | Molecule: 18 ("c1(c(nccc1)N)OCc2ccccc2") |
99 | 0 |
| 19 | Molecule: 19 ("c1(ccc(cc1O)OCC=C)C(c2ccccc2)=O") |
99 | 72 |
| 20 | Molecule: 20 ("c1(ccc(cc1)OC)C(O)=O") |
99 | 0 |
a For entries 1-10 we have proven that [TMAH][Al2Cl7] works equally well as [BMIM][Al2Cl7]
b The conversion was determined after workup by GC and NMR spectroscopy. * (not displayable in SMILE, 17O was used instead) Indicates which methoxyl group is cleaved predominantly in the dimethoxyaromatic compounds. The ratio of regioisomers is shown in brackets below the conversion.
[TMAH][Al2Cl7] (1)
Aluminum chloride (173 g, 1.3 mol) was suspended in dichloromethane. The suspension was cooled with an ice bath, and trimethylammonium chloride (62 g, 0.65 mol) was added with stirring. After the addition, the reaction mixture was warmed to ambient temperature and was stirred for 2 h. The clear yellow solution of ionic liquid could be used as such. To obtain the ionic liquid in a pure form, the dichloromethane was evaporated. Yield: 235 g (99%).
The density of the liquid was determined by accurately measuring the weight of 10 mL of sample, and was found to be 1.23 g/mL.
Laboratory Experiments. General Procedure for Entries 1-20 of Table 2
The substrate was dissolved in dichloromethane (1 g/40 mL). To the stirred solution, ionic liquid (3 equiv. of [TMAH]-[Al2Cl7]) was added dropwise. The reaction mixture was warmed to reflux. The reactions were monitored by GC and TLC. After the reaction was complete, the mixture was poured into dilute hydrochloric acid (1 M). The aqueous layer was extracted three times with ethyl acetate. The ethyl acetate was washed with saturated NaHCO3 and brine, and then the ethyl acetate was dried with MgSO4 and the solvents were evaporated.
In cases where the substrate had basic functional groups, the reaction mixture was poured into water. The aqueous layer was neutralized with a NaOH solution (1 M). The aqueous layer was extracted three times with ethyl acetate, and then the ethyl acetate was washed with brine, dried with MgSO4, and the solvents were evaporated.
N-Butyl-N-methylimidazolium Chloride
A mixture of N-methylimidazole (100 g, 1.22 mol) and n-butyl chloride (113 g, 1.22 mol) was stirred and heated at 70 °C for 96 h. The reaction mixture was cooled to ambient temperature. The viscous oil was washed three times with ethyl acetate and then the ethyl acetate remaining was removed in vacuo. The resulting oil crystallized as a white solid. Yield: 210 g (98%)
[BMIM][Al2Cl7] (2)
N-Butyl-N-methylimidazolium chloride (5 g, 28.6 mmol) was stirred under a stream of nitrogen gas while being cooled with an ice bath. Aluminium chloride was added in small portions to the stirred powder. A highly exothermic reaction took place. After all the aluminium chloride (7.1 g, 53.2 mmol) had been added, the cooling was removed and the reaction mixture was warmed to ambient temperature. This process yielded a yellow liquid (12.1 g, 99%). The density of the liquid was determined by accurately measuring the weight of a 10 mL sample, and was found to be 1.15 g/mL.
[BMIM][Al2Cl7] could also be prepared using dichloromethane as a solvent, as described above for [TMAH][Al2Cl7].
4-Hydroxy-5-methoxyindanone
Large-Scale Procedure: A solution of [BMIM][Al2Cl7] (250 mL, 2.5 equiv.) in dichloromethane (1 L) was added dropwise to a solution of 4,5-dimethoxyindanone (50 g, 0.26 mol) in dichloromethane (500 mL). The reaction mixture was stirred for 23 h at 40 °C. The reaction mixture was poured into a mixture of water (4 L) and concentrated hydrochloric acid (0.6 L). The aqueous layer was extracted three times with dichloromethane. The organic layer was subsequently dried with MgSO4 and the solvents were evaporated. This process yielded the crude product (45 g, 97%). After recrystallization from a mixture of heptane and ethyl acetate (3:2), the purified product (37 g, 80%) was obtained. The product had a purity of 99.2% according to HPLC. NMR (CDCl3) was in agreement.
This procedure was also followed using [TMAH][Al2Cl7] (191 mL, 2.5 equiv.), which gave a similar result.
6,7-Dihydroxytetralone
[TMAH][Al2Cl7] (2.5 mL) was added to (3,4-dimethoxyphenyl)butanoic acid (200 mg, 0.893 mmol). No cyclization was observed by TLC after 1 h of reaction time. The temperature was raised to 60 °C, but again no cyclization was observed at this temperature. The solution was then heated to 150 °C overnight. The reaction mixture was cooled and hydrochloric acid (1 M, 15 mL) was added. The reaction mixture was stirred for 30 minutes. The aqueous layer was extracted three times with ethyl acetate. The ethyl acetate phase was washed with saturated aqueous NaHCO3 and brine, dried with MgSO4, and then the solvents were evaporated. This process yielded 6,7-dihydroxytetralone (80 mg, 50%), as was confirmed by NMR spectroscopy (CDCl3 or CD3OD).
The candle that burns twice as bright burns half as long
(Hive Bee)
05-02-04 09:08
No 504369
(Rated as: excellent)
A new method for the prepartion of organic iodides
Herman Stone, Harold Shechter
J. Org. Chem., 1950, 15(3), 491-495
No DOI found

[...]
Phosphoric acid and potassium iodide are of value in that they serve as a convenient, readily available source of hydrogen iodide, and advantage is taken of the acid-catalyzed solvolytic cleavage of ethers by strong acids (13). Cleavage of simple aliphatic ethers by these reagents is illustrated by the following equation :
R-O-R + 2 KI + 2 H3PO4 --> RI + KH2PO4 + H2O
In initial experiments (Table I) with dibutyl ether, potassium iodide, and excess 50% phosphoric acid at 110° it was found that no reaction occurred. When the concentration of the phosphoric acid was increased to 85% by weight and finally to an optimum concentration of 95%, cleavage of the ether proceeded rapidly, with minimum reduction and dehydration, to give 1-iodobutane in 75.1% and 81.0% yields respectively. Since anhydrous phosphoric acid and tetraphosphoric acid were found to have marked dehydrating action and hydrogen iodide is relatively insoluble in these solvents, a general routine procedure using excess 95% orthophosphoric acid was adopted. Sodium iodide could be substituted for potassium iodide without appreciably altering the reaction efficiency.
Upon extension of the method to various aliphatic and alicyclic ethers (Table II), it was found that the procedure was generally applicable and had many advantages over previous methods. Cleavage of the mixed aryl-alkyl ether, beta-naphthyl ethyl ether, into beta-naphthol and iodoethane occurred readily, but the reagent had no effect on diphenyl ether. No attempt was made to obtain maximum yields in this series; however, the reaction was accelerated and made almost quantitative by increasing the mole ratio of potassium iodide to ether.
[...]
Table II
Cleavage of ethers with potassium iodide and 95% phosphoric acida
| Compound | Time, hrs | Conversion to iodide, % | Yield of iodide, % |
| Dibutyl ether | 5 | 77.7b | 81.0 |
| Diisopropylether | 3 | 68.7 | 89.8 |
| Tetrahydrofuran | 5 | 96.0 | 96.0 |
| Diphenylether | 6 | 0 | 0 |
| beta-Napthyl ethyl ether | 3 | 60.5 | 77.7c |
a Experiments were conducted with an ether, potassium iodide, and 95% phosphoric acid mole ratio of 1:4:5.9 at the reflux temperature of the reaction mixture. With dibutyl and diisopropyl ethers, mole ratios of 1:3.5:5.3 and 1:6:8.7 were used.
b A 7.3% yield of l-butanol was obtained.
c A yield of &naphthol of 89.9% was obtained.
[...]
Experimental
Since the procedures for the reactions of potassium iodide and orthophosphoric acid with various ethers, alcohols, and olefins are essentially the same, a detailed procedure is described for only a single member of each class of compounds.
[...]
Phosphoric acid of the desired concentration was prepared by adding the calculated quantity of 85% phosphoric acid to either water or phosphoric anhydride with stirring and cooling. The quantity of potassium iodide varied from 1.5-3.0 times the theoretical. The mixture was cooled before adding the potassium iodide to prevent evolution of hydrogen iodide and oxidation to iodine. The reaction proceeded more rapidly with large excesses of potassium iodide. Separation of the reaction products was accomplished either with a precision rectifying-column or a modified Claisen distilling-flask at various pressures.
[...]
Dibutyl ether, potassium iodide, and 96% phosphoric acid.
Orthophosphoric acid (85%; 346 g.; 202 ml; 3.0 moles) was added, with stirring, to 79 g. of phosphoric anhydride (=95% phosphoric acid) in a dry 1-l three-necked flask equipped with a sealed stirrer, a reflux condenser, and a thermometer. After the mixture had cooled to room temperature, 445 g. (2.68 moles) of potassium iodide and 100 g. (0.77 moles) of dibutyl ether (b.p. 139.5-140.5°) were added. The mixture was stirred and heated at reflux temperature for five hours during which time a dense oil separated from the acid layer. The stirred mixture was cooled to room temperature, and 150 ml. of water and 250 ml. of diethyl ether were added. The ether layer was separated, decolorized with sodium thiosulfate solution, washed with a cold saturated sodium chloride solution, and dried over sodium sulfate. The ether was evaporated, and the mixture was rectified at atmospheric pressure in a fourfoot rectifying-column packed with glass-helices. Three fractions were obtained:
(a) an azeotrope (19 g.; b.p. 110°) consisting of 11 g. of 1-iodobutane and 8 g. of 1-butanol,
(b) 1-iodobutane (200 g.; b.p. 129.5-30.5°; n20D 1.504; d204 1.630), and
(c) dibutyl ether (4 g.; b.p. 140°; n20D 1.402; d204 0.770).
Yield of 1-iodobutane; 77.7%
[...]
This article has already been mentioned: Post 108406 (dormouse: "Diethylamine -Baxter", Novel Discourse)
The tendency is to push it as far as you can
(Hive Bee)
05-08-04 18:17
No 505891
(Rated as: good read)
Application of Ionic Liquid Halide Nucleophilicity for the Cleavage of Ethers:
A Green Protocol for the Regeneration of Phenols from Ethers
Shanthaveerappa K. Boovanahalli, Dong Wook Kim, and Dae Yoon Chi
J. Org. Chem., 2004, 69(10), 3340-3344
DOI:10.1021/jo035886e

Abstract
We have used the high nucleophilicity of bromide ion in the form of the ionic liquid, 1-n-butyl-3-methylimidazolium bromide ([bmim][Br]), for the nucleophilic displacement of an alkyl group to regenerate a phenol from the corresponding aryl alkyl ether. Using 2-methoxynaphthalene (1) as a model compound, we found that the combination of ionic liquid [bmim][Br] and p-toluenesulfonic acid with warming effected demethylation in 14 h, affording the desired product 2-naphthol (2) in good yield (97%). Various other protic acids (MsOH, hydrochloric acid (35%), dilute sulfuric acid (50%)) could be used as a proton source in this demethylation reaction. Under the same conditions, cleavage of alkyl alkyl ether 2-(3-methoxypropyl)naphthalene yielded mixture of corresponding 2-(3- bromopropyl)naphthalene and 2-(3-hydroxypropyl)naphthalene. Dealkylation of various aryl alkyl ethers could also be achieved using significantly reduced (i.e., stoichiometric) amounts of concentrated hydrobromic acid (47%) in the ionic liquid. Both procedures afforded the desired products in moderate to good yield; however, cleavage of aryl alkyl cyclic ether, 2,3-dihydrobenzofuran, resulted in low yield of the desired product o-2-bromoethylphenol. The convenience of this method for ether cleavage and its effectiveness using only a moderate excess of hydrobromic acid make it attractive as a green chemical method.

[...]
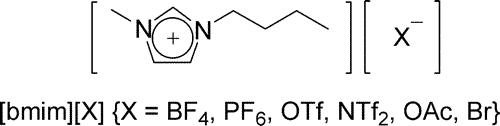
Figure 1 Ionic liquids.
TABLE 1. Demethylation of 2-Methoxynaphthalene with Various Protic Acids in [Bmim][BF4]a

| Entry | Protic acid | Equiv. | [bmin][Br] (equiv.) | Time (h) | Yield (%)b |
| 1 | HBr (47%) | 5 | 7 | 96 | |
| 2 | HBr (47%) | 2 | 9 | 97 | |
| 3 | HBr (47%) | 1 | 32 | 94 | |
| 4 | 3 | 22 | |||
| 5 | p-TsOH | 3 | 22 | 30 | |
| 6 | p-TsOH | 2 | 2 | 22 | 97 |
| 7 | p-TsOH | 3 | 3 | 14 | 97 |
| 8 | MsOH | 3 | 22 | 27 | |
| 9 | MsOH | 3 | 3 | 14 | 97 |
| 10 | AcOH | 3 | 22 | ||
| 11 | AcOH | 3 | 3 | 22 | |
| 12 | HCl (35%) | 3 | 22 | 25 | |
| 13 | HCl (35%) | 3 | 3 | 22 | 93 |
| 14 | H2SO4 | 3 | 22 | 29 | |
| 15 | H2SO4 | 3 | 3 | 22 | 93 |
| 16 | H2O | 3 | 3 | 22 |
a All reactions were carried out on a 1.0 mmol reaction scale of 2-methoxynaphthalene 1 in 1.0 mL of [bmim][BF4] at 115 °C.
b Isolated yield.
[...]
TABLE 2. Conversion of 2-Methoxynaphthalene to 2-Naphthol in Various Ionic Liquids and Solventsa
| entry | [bmim][X] or solvent | protic acid (equiv.) | [bmim][Br] (equiv.) | time (h) | yield (%)b |
| 1 | [bmim][PF6] | HBr (47%) (2) | 12 | 90 | |
| 2 | [bmim][NTf2] | HBr (47%) (2) | 16 | 92 | |
| 3 | [bmim][OTf] | HBr (47%) (2) | 12 | 95 | |
| 4 | [bmim][OAc] | HBr (47%) (2) | 22 | 37 | |
| 5 | ClCH2CH2Cl | HBr (47%) (2) | 48 | 37 | |
| 6 | benzene | HBr (47%) (2) | 48 | 34 | |
| 7 | CH3CN | HBr (47%) (2) | 48 | 19 | |
| 8 | H2O | HBr (47%) (2) | 48 | 35 | |
| 9 | [bmim][PF6] | p-TsOH (3) | 3 | 20 | 90 |
| 10 | [bmim][NTf2] | p-TsOH (3) | 3 | 16 | 93 |
| 11 | [bmim][OTf] | p-TsOH (3) | 3 | 22 | 93 |
| 12 | [bmim][OAc] | p-TsOH (3) | 3 | 2 | 2 – |
a All reactions were carried out on a 1.0 mmol reaction scale of 2-methoxynaphthalene 1 in 1.0 mL of ionic liquid or solvent at 115 °C.
b Isolated yield.
[...]
TABLE 3. Cleavage of Various Ethers in [Bmim][BF4]a
| Entry | Substrate | Method Aa time (h) |
Method Aa yield (%)c |
Method Bb time (h) |
Method Bb yield (%)c |
| 1 | Molecule: 1 ("c12ccccc2ccc(c1)OCCC") |
13 | 95 | 20 | 91 |
| 2 | Molecule: 2 ("c13ccccc3ccc(c1)OCc2ccccc2") |
4 | 93 | 4 | 90 |
| 3 | Molecule: 3 ("c12ccccc2cccc1OC") |
12 | 89 | 20 | 89 |
| 4 | Molecule: 4 ("c21cc(ccc1oc3c2cccc3)OC") |
5 | 94 | 10 | 95 |
| 5 | Molecule: 5 ("c21ccc(cc1ccc(c2)Br)OC") |
10 | 95 | 12 | 92 |
| 6 | Molecule: 6 ("c1(ccc(cc1)OC)C(c2ccccc2)=O") |
13 | 93 | 13 | 91 |
| 7 | Molecule: 7 ("c1(ccccc1)c2ccc(cc2)OC") |
20 | 94 | 20 | 95 |
| 8 | Molecule: 8 ("c21ccc(cc1CCC3C2CCC4(C3CCC4=O)C)OC") |
5 | 86 | 5 | 85 |
| 9 | Molecule: 9 ("c12ccc(cc1CCC3C2CCC4(C3CCC4O)C)OC") |
5 | 87 | 5 | 87 |
| 10 | Molecule: 10 ("c\21ccc(cc1OC(/C=C/2)=O)OC") |
13 | 75 | 13 | 80 |
| 11d | Molecule: 11 ("c12ccccc2ccc(c1)CCCOC") |
12 | 46 | 12 | 47 |
| 12e | Molecule: 12 ("c21ccccc1OCC2") |
13 | 40 | 13 | 40 |
a All reactions were carried out on a 1.0 mmol reaction scale of ether in 1.0 mL of [bmim][BF4] at 115 °C using a 2.0 mmol ratio of concentrated hydrobromic acid (47%).
b All reactions were carried out on a 1.0 mmol reaction scale of ether in 1.0 mL of [bmim][BF4] at 115 °C using a combination of p-TsOH and [bmim][Br] each in a 3.0 mmol ratio.
c Isolated yield.
d 2-(3-Bromopropyl)naphthalene was also obtained in 45 and 46% yield for method A and method B, respectively.
e 2-(2-Bromoethyl)phenol was obtained, and starting material was recovered.
[...]
Experimental Section
Typical Procedure for Cleavage of Ether: Method A.
Ether (1 mmol) and concentrated hydrobromic acid (47%, 2 mmol) in 1-n-butyl-3-methylimidazolium tetrafluoroborate (1.0 mL) were stirred at 115 °C for an appropriate time (see Table 3). The reaction time was determined by TLC analysis. The reaction mixture was extracted with diethyl ether (4 x 10 mL).
The combined ether extracts were concentrated under reduced pressure, and the resulting product was purified by flash column chromatography (10% EtOAc/hexane) to furnish phenol.
The products were characterized by comparison of their 1H NMR, 13C NMR, and TLC data with authentic samples. The spectral data of all products were identical with those of authentic samples.
Method B.
Ether (1 mmol), 1-n-butyl-3-methylimidazolium bromide (657 mg, 3 mmol), and p-TsOH (3 mmol) in 1-n-butyl-3-methylimidazolium tetrafluoroborate (1.0 mL) were stirred at 115 °C for an appropriate time (see Table 3). The reaction mixture was worked up as in method A to obtain the phenol product.
[...]
The tendency is to push it as far as you can
(Chief Bee)
07-12-04 11:07
No 518930
(Rated as: excellent)
Conception, Characterization and Correlation of New Marine Odorants
Philip Kraft, Walter Eichenberger
European Journal of Organic Chemistry, No. 19, pp. 3735-3743 (2003) (../rhodium/pdf /eugenol.deme
[...] In the next step, the methyl ether protecting groups of 5,6-dimethoxy-1-methylindane (10) had to be cleaved. For this we decided to employ trimethylsilyl iodide (TMSI)7 in acetonitrile.8 The cleavage of 10 with TMSI was conducted at room temp. to afford after the usual workup and purification by silica-gel FC in 93% yield 1-methylindan-5,6-diol (11). This diol 11 had already been reported by Ayer and Singer9 as the unintended product of an attempt to correlate 4-(3,4-dihydroxyphenyl)butan-2-one with zingerone (vanillyl acetone) by demethylation of the latter with TMSI. Yet, the reported yield was only about 30%, and the synthesis is less practical and less generally applicable than our approach. The spectroscopic data of 11 prepared on our route matched with those reported by Ayer and Singer.9
[...] The allyl analogue 27 was synthesized from eugenol by cleavage of the phenolic methyl ether group employing lithium chloride in refluxing DMF according to a method of Piras and co-workers.20 4-Allylpyrocatechol was obtained in 50% yield after 44 h reaction time, usual workup and purification by silica-gel FC.
Experimental
Demethylation of 5,6-dimethoxy-1-methylindane (10) with TMSI
At room temp. under N2, Me3SiI (TMSI, 27.5 mL, 202 mmol) was added dropwise with stirring in the course of 90 min into a solution of 10 (19.4 g, 101 mmol) in MeCN (150 mL). Stirring was continued at room temp. for 2.5 days, with an additional quantity of Me3SiI (TMSI, 10.0 mL, 73.5 mmol) being added after 48 h. The reaction mixture was poured into water (500 mL), and extracted with Et2O (2 × 200 mL). The combined extracts were washed with 40% aq. NaHSO3 (100 mL), water (100 mL) and brine (50 mL), dried (Na2SO4) and concentrated in a rotary evaporator. Silica-gel FC (pentane/Et2O, 2:1, Rf = 0.28) furnished 1-methylindan-5,6-diol (11, 15.5 g, 93%), the spectroscopic data of which matched with those reported9.
Demethylation of Eugenol to 4-allylpyrocatechol with LiCl/DMF
LiCl (292 g, 6.89 mol) was added to a solution of eugenol (354 mL, 2.30 mmol) in DMF (3.7 L), and the mixture was refluxed for 44 h, with additional portions of LiCl (292 g, 6.89 mol) being added after 4 h, 22 h and 29 h. The reaction mixture was allowed to cool down to room temp., and diluted with toluene (2 L). The formed precipitate was filtered off and washed with toluene, the washings were combined with the organic solution and concentrated in a rotary evaporator. Silica-gel FC (Et2O/pentane, 1:1, Rf = 0.37) provided 4-allylpyrocatechol (173 g, 50%).
References
[7]
Quantitative dealkylation of alkyl ethers via treatment with trimethylsilyl iodide. A new method for ether hydrolysis.
Jung, Michael E.; Lyster, Mark A.
Journal of Organic Chemistry 42, 3761-4 (1977) (../rhodium/pdf /ether.cleava
Abstract
PhOMe was kept 21 h at 50° in CDCl3 containing a 0.3-fold molar excess of Me3SiI to give 100% PhOSiMe3 and 100% MeI. The cleavage of 25 other ethers is reported under these conditions. Me3SiI is prepared by refluxing (Me3Si)2O with Al and I. The mechanism of the ether cleavage is discussed.
____ ___ __ _
[8]
Synthetic methods and reactions. 62.
Transformations with chlorotrimethylsilane/sodium iodide, a convenient in situ iodotrimethylsilane reagent.
Olah, George A.; Narang, Subhash C.; Gupta, B. G. Balaram; Malhotra, Ripudaman
Journal of Organic Chemistry 44, 1247-51 (1979) (../rhodium/pdf /ether.cleava
Abstract
A new, convenient, inexpensive alternative to Me3SiI reagent is explored. A mixture of Me3SiCl-NaI in MeCN is a better reagent than Me3SiI for the cleavage of esters, lactones, carbamates and ethers. Cleavage of esters and lactones (10 examples) occurred somewhat slower with the present system than with Me3SiI. On the other hand, ethers (7 examples) cleaved much more readily with the present system. A feasible mechanism is proposed for this disparity. Carbamates (6 examples) also underwent facile cleavage to give the corresponding amines. The general applicability of the method was shown using various types of substrates. The facile conversion of alcs. to iodides using the present method is also reported. Conversion of alcs. to iodides is much faster with Me3SiCl-NaI than with Me3SiI, and iodides are formed in excellent yield.
____ ___ __ _
[9]
Phenolic metabolites of the bird's nest fungus Nidula niveo-tomentosa
William A. Ayer and Peter P. Singer, Phytochemistry 19(12), 2717-2721 (1980)
DOI:10.1016/S0031-9422(00)83949-7
Abstract
The metabolites of the bird's nest fungus Nidula niveo-tomentosa have been examined. Niduloic acid (3-hydroxy-5-(p-hydroxyphenol) pentanoic acid) is a new natural product. 4-(p-Hydroxyphenyl)-2-butanone, 4-(p-hydroxyphenyl)-2-butanol, trans-4-p-hydroxyphenylbut-3-en-2-one, 4-(3′,4′-dihydroxyphenyl)-2-
____ ___ __ _
[20]
Dealkylation of Activated Alkyl Aryl Ethers Using Lithium Chloride in Dimethylformamide
Angela M. Bernard, M. Rossella Ghiani, Pier Paolo Piras, Antonio Rivoldini
Synthesis 287-289 (1989) (../rhodium/pdf /ether.cleava
Abstract
Alkyl aryl ethers having electron-withdrawing substituents in the ortho or para positions are easily cleaved with lithium chloride in dimethylformamide.
Oops, apparently already posted in Post 444300 (Lego: "Dealkylation with LiCl/DMF", Methods Discourse)
The Hive - Clandestine Chemists Without Borders