(Official Hive Translator)
08-16-01 02:33
No 205055
(Rated as: excellent)
This is another synthesis from "the Russian Hive" - hyperlab.org. It describes prepn of P2P from nothing else but benzene, acetone and Mn(CH3COO)3. The latter is easily prepared from KMnO4(see below). As far as the search engine goes, it hasn't been covered here at all (xcept for one unanswered post in Aquisition where some stranger asks where to buy manganese triacetate
Here's the deal:
15 ml benzene, 15 ml acetone, 25 ml AcOH and 5 mmol(1,01 g) Mn(AcO)3 are boiled under N2 atmosphere until the color of the soln changes from dark brown Mn(AcO)3 to pink Mn(AcO)2 - approx. 1,5 h. The rxn mixtr is diluted w/40 ml ether, washed w/25 ml water and 2x25 ml 5% NaHCO3, the org layer is dried and evap'd to yield 40% P2P (134 mg)
Now, as you can see the yield is not that great - but, hey, who cares for acetone, what is more important the benzene/acetone to Mn(AcO)3 ratio is huge. Well, i can only say that the above procedure (as i have some good reasons to believe) bears rather analytic than preparative approach and the amt of Mn(AcO)3 undoubtedly can be scaled up significantly, but the exact answer is still to be found.
Although SWIM didn't succeed w/this rxn the 1st and the only one thus far time he tried it (see the experimental details below) there can be no doubt that it works as i've personally read two articles dedicated to studying its mechanism. It works even better w/substituted benzenes: for anisole the yield is 74% (in 45 min), unfortunately a mixture of isomers is formed of which ortho- predominates. Para-dimethoxybenzene is even more activated than anisole and completely symmetric, it seems to bee ideal for this rxn, don't you think so, bees?
The rxn also runs w/naphtalene (selectively forming 1-acetonylnaphtalene), furan, sylvan, pyridine, thiophene (all forming alpha-acetonyl compds), chloro- and cyano-benzene, toluene (forming a mxtr of isomers) and, beyond a shadow of a doubt, a whole lot of other aromatics which opens so many roads to new, untasted yet compounds - (which, coincidentally, are currently being discussed in Serious Discourse/Fluoroamph. thread) - that it is even scary. Just needs to be made to work
The mechanism of the rxn is as follows: first a ÑÍ2=Ñ(ÑH3)-Î-Ìn(3)-ÀãÍ complex is formed, which then falls apart w/simultaneous cleavage of Mn-O bond, acetonyl combines w/aromatic nucleus (the limiting step) and the resulting adduct radical is rapidly reduced w/another Mn(AcO)3 molecule.
Well, with that said let me finally get to the preparation of Mn(AcO)3 - i'll skip the abstract and just tell you guys what SWIM did, from the beginning to the end.
KMnO4 is reduced w/hexamine and HCl to MnCl2, precip'd as the carbonate, converted to Mn(AcO)2 and oxidized w/KMnO4 in boiling GAA to Mn(AcO)3.
15 g KMnO4 was dissolved in some hot water and combined w/a soln of 15 g camping fuel (SWIM used hexamine cause he had it at hand - any ammonium salt will do just fine). Nothing happened. Then conc HCl was added in portions w/swirling until mixtr acidic. As he did it, the mxtr gradually turned completely clear and the stench of formaldehyde became almost unbearable
When all settled, the water was decanted into another jar (to collect the still remaining suspended MnCO3 later) and the precip't washed in the same manner once again, MnCO3 in the other jar washed later too. Then all combined, water decanted off, precip't vac. filtered, DO NOT DRY IT - there's no need for that, and it WILL oxidize somewhat during drying. Just scrape it off the filter and dump into some GAA. Now, guess what, there was no fizzling and bubbling at this stage as SWIM expected, in fact, to dissolve all the MnCO3 it took a 2 hour reflux. At this stage the soln was a pleasant pink color, just as it should have been.
The GAA was boiled off overnight at 130 C in an oil bath and when SWIM woke up he found that the bottom of the flask was covered w/some white (not pink) crystals - well, he thought, that must be unhydrous Mn(AcO)2 (the pink stuff is a tetrahydrate) - and so it turned out to be. SWIM dosn't remember the exact weight (uhhhh... 21 g?) but the yield was quantative.
Now SWIM went on to dissolving his salt in GAA, 250 ml (SWIM thought you'd have to 1st evap. GAA and then dissolve in it again as the former one has water in it - but he was wrong as you'll see. Well, he needed to weigh it anyway. Just skip this evap'n/dissolution step, it's probably not needed) - the shit doesn't dissolve! even w/reflux! Probably, SWIM thought, the unhydrous stuff doesn't, so he added a theoretical amt of water, well, a little more in fact, about 7 ml to the mxtr and w/some boiling most of it went in the soln, some was still left at the bottom - it was OK, as it turned.
To this boiling soln SWIM added about 5,4 g (~1/4 molar equivalent) of KMnO4, in, say, 6 portions. During and after each addition the mxtr was vigorously stirred w/a glass rod. The KMnO4 must be prior to that ground as fine as possible - it's not hard at all. The mxtr turned dark and opaque. Boiling was continued for some 10 min, then the flask put into the fridge (16 C) and 3 ml water added to it to induce crystallization. The walls of the flask were periodically furiously scratched (on the in-side
The mxtr was filtered, the mother liquor still very dark (put 3 ml water into it and let stand for a week - all of your prouct will crystallize and the liquor will turn colorless, this time the crystals will be not dark brown, but of rust/cinnamon powder color, just like the dry stuff), crystals sucked as dry as possible and dried for ~3 days in a dessicator over CaO (SWIM doesn't have one so he designed one from a saucepan, a couple of plates, plastic bag and a heavy hammer
OK bees i gotta go now, tomorrow i'll post the details of SWIM's P2P fuckup and maybe someone will tell me what was the reason (frankly speaking, i think it was his fucken carelessness and dirty reagents)
Hope this one will draw some attention
Antoncho
(Chief Bee)
08-16-01 04:27
No 205063
This is GREAT! This synthesis has been covered in Fester's book earlier, but it was ridiculed, because with the price of Mn(III)Acetate ($500 for 250g), it would be cheaper to buy meth on the street. Now with a preparation of that using simple reagents, this could actually be of use! Thanks Antoncho!
But - The mechanism of the rxn is as follows: first a ÑÍ2=Ñ(ÑH3)-Î-Ìn(3)-ÀãÍ complex is formed - this cannot be read with my font. Could you rewrite that using non-kyrillic characters?
I believe the refs for this rxn is Chemical Abstracts 77, 151620 (1972), JACS 93, 524-527 (1971) and Bulletin of the Academy of Science of the USSR 21(7) 1626 (1972).
(Official Hive Translator)
08-16-01 08:20
No 205112
a) thank you , chief, thanks and thanks and thanks a lot for your praise :):):)
b) yesss', it's "The mechanism of the rxn is as follows: first a CH2=C(CH3)-O-Mn(3)-ArH complex is formed "... et cetera. Always forget there are some shortcuts from the cyrillic keyboard to the chemical nomenclature
c) now comes the time for the details on the final experiment.
To a soln of 120 ml benzene, 80 ml acetone (both fucken tech grade - yess', i'know, the amt of the impurities in them, multiplied by their _huge_ quantities... and the other stuff... you wouldn't believe how vague is the borderline between "chem. pure" and "tech grade" in the FUCKCKCKEN, in this fucken, fucken country is!! - i should have distilled them ANYWAY, shouldn't i? and 150 ml GAA there was added 10 g Mn(AcO)3, w/out any stirring (turned to be fine) and the mxtr brought to a boil for ~ 1,5 h. By the end of that period (y'know, i actually think that it stayed dark brown for an hour and then suddenly beecame pink in a matter of like 15-20 min's) the mxtr diluted w/500 ml H2O, the aq layer extr'd w/80 ml more benzene (yellowed quite considerably), the org. layers washed w / aNaHCO3 and combined and washed w/a bisulfite reagent.
The latter was prep'd from : 5g Na2SO3 ; 1/2 mol. eq. CH3COOH; denat. alcohol - all quite in accordance w/ the Eleusis method, xept NaHSO3 is prep'd in situ w/Na2SO3 and CH3COOH. Do you think it makes a difference? My comrades :) have isolated P2P this way many times...
And - ALAS - the bisulfite wash yielded naught but some vagueness in the aq. layer, which didn't even .... ahhhhhh.... i'm more of a curious chemist than a desperate speedhead, yes, but..... it's very disappointing. Oh... Fuck it. Currently, it's evaporating on a 125 C oil bath.
Now that the fucken brew is finally evap'd i want to tell you that:
a) there IS a 1 ml (just as there should have been) of an unevaporated liquid in the evap's basseign.
b) Can there be a possibility that the bisulfite agent doesn't work all the time - i have a goood, real good reason to believe this - ESPECIALLY when the concentration of the product is less than ...
c) i'm so fucken unexperienced in making a bisulfite!! please, ANY help!!! Very, very appreciated!
Oh, meeen. Bees!
Antoncho
(Hive Bee)
08-16-01 09:24
No 205132
Usually, one isolates the organic product before attempting to make the bisulfite addition product. Some impurities interfere with the reaction, unfortunately. Have you looked at: ../rhodium /eleusis
(Chief Bee)
08-16-01 11:14
No 205163
For workup, I would dilute with a lot of water like you say you did, then extract that into any non-polar solvent, wash the combined organic layers with water, aq NaHCO3, and finally with a saturated NaCl solution. Then I would dry the organic layer over MgSO4, and vacuum distill (preferably with a column, but you can do without). After the benzene has distilled over, the P2P should come next, and what's left in the boiling flask is just crap.
I think that bisulfite purification is killing yields considerably and that the procedure is way overrated.
(Wizard Master)
08-23-01 20:54
No 206943
The Mn(CH3COO)3 can be made electrochemically and can be regenerated insitu during the reaction of the P2P synthesis.
(Hive Addict)
08-23-01 21:25
No 206951
hey Rhodium, this looks like it could be a new link to add to your site- when you get time of course.
(Chief Bee)
08-24-01 01:27
No 206992
Of course. I have a 5 MB directory on my HD with very good stuff to upload already, I just want it to be nicely edited first. I just need the time to do it. If I hadn't so much to do out in the ordinary world, the Hive and my page could easily be a full-time job. I also have 10-20 kilograms of copied references I could do something nice with, if I had the time to scan and OCR it...
I am in favor of cloning. I really need a spare Rhodium to do all this for me... I have a lot of things that just need to be typed up from refs, interested people can contact me, And I'll send you scanned TIFFs for you to convert into ASCII. I can then do the editing/HTML myself.
(Official Hive Translator)
08-25-01 07:11
No 207319
Dear WizardX!
Are you sure that it can be electrochemically made? If so, do you know the precise cond's (like voltage and temp) ?
If this worked, it would bee a real breakthrough in meth production!
Antoncho
(Master Searcher)
08-25-01 16:40
No 207386
I'd be interested in that too (electrochemical process for Mn(III)acetate). I looked at every patent in 205/440 at www.uspto.gov and didn't find it. I also looked at the classification manual at class 205 and 423 and can't seem to find a good home for it. I did find some interesting patents while searching and doing keyword searches:
3546082 electrolytic process for Pd acetate, regeneration of Wacker catalysts
3969405 which includes the lines:
Among the metal compounds capable of oxidizing hydrocarbon or other organic substances, the cobalt (III) and manganese (III) salts of carboxylic acids are preferred. They are powerful oxidizers; they are satisfactorily soluble in such organic solvents; and they are easily produced by known methods. From these points of view, the cobalt (III) and manganese (III) salts of fatty acids containing from 2 to 10 atoms of carbon, and preferably their acetates, are particularly satisfactory. For example, cobalt (III) acetate may be produced by co-oxidation of cobalt (II) acetate with acetaldehyde in acetic acid in the presence of oxygen. Manganese (III) acetate may be produced by oxidation of manganese (II) acetate with potassium permanganate in acetic acid. The cobalt (III) and manganese (III) salts of the other fatty acids can be produced in analogous manner or by exchange reaction between these and cobalt (III) acetate.
4297520 anisaldehyde from p-methoxytoluene
http://www.geocities.com/dritte123/PSPF.
(Newbee)
08-25-01 17:51
No 207388
Would it be safe to assume that one could substitute methylene-dioxy-benzene for benzene, achieving MDP-2P respectively? Or would the MD interfere with this reaction? I would love this to be true, but i haven't the slightest clue if it would make a difference. I certainly hope not...
And if it would react, Id think that isomerism would be a problem, also. Any thoughts?
Of COURSE we don't know what we're doing! That's why it's called research! (boom...) :)
(Wizard Master)
08-26-01 19:41
No 207636
I'll put some ref's together, and I'm doing a major upgrade at my Wizard X site with 10 new XFiles.
(Wizard Master)
08-26-01 19:48
No 207639
Antoncho: Yes, it will be a major breakthrough in meth production, you can even make PAA, phenylacetic acid.
But, I can see the narcs at this site posing as user-bees quickly jumping on this.
(Official Hive Translator)
08-27-01 09:02
No 207730
Yes, it will be a major breakthrough in meth production, you can even make PAA, phenylacetic acid
Dear WizardX! I too have been thinking of what other things may bee used in this rxn instead of acetone. As i understand it, acetone is suitable because =O draws electrons from a-carbons, making them easily deprotonated.
If so, then why not use acetaldehyde? If it worked, it could bee an awesome route to all the 2C-...'s: 2,5-dimethoxybenzene (or -phenylmercaptan - see my thread in Novel discourse) ---> 2,5-dimethoxyphenylacetaldehyde ---> (Leuckart) 2C-H.
What do the bees think about this?
Antoncho
(Stoni's sexual toy)
08-27-01 10:55
No 207756
> acetone is suitable because =O draws electrons from
> a-carbons, making them easily deprotonated.
Ever heard of keto/enol-tautomerism?
(Stoni's sexual toy)
08-28-01 12:53
No 208067
Oooops, sorry, brainfart on my part. Somehow I thought the equilibrium would be completely on the side of the enolate.
(Wizard Master)
08-28-01 20:07
No 208201
From memory,(without having my Spellbook with me), the mechanism is.....
CH3COOH ==>> .CH2COOH Radical generated by Mn(AcO)3
.CH2COOH + C6H6 ==>> C6H5CH2COOH
(Master Searcher)
09-01-01 12:20
No 209319
Here's some more US patents relating to the Mn(OOCCH3)3 process.
4011239 olefins + ketones, etc.
3927077
3860612
4,230,893:
4-Hydroxy-3-methoxyphenylacetone, an intermediate for methyldopa, has been prepared from reacting two moles of guaiacol and one mole of a 2-alkoxy propanol followed by basic elimination to form the enol ether of 4-hydroxy-3-methoxy phenyl acetone. The enol ether is subsequently converted to 4-hydroxy-3-methoxy phenyl acetone upon treatment with a strong acid.
http://www.geocities.com/dritte123/PSPF.
(Newbee)
09-17-01 21:39
No 214603
In response to:
"interested people can contact me, And I'll send you scanned TIFFs for you to convert into ASCII. I can then do the editing myself."
I am very intrested in helping out I have state of the art OCR SW and if you like I can give you a place to ftp the files to?
Thanks
[quote]
Hum did you get that?
(Hive Bee)
09-18-01 08:00
No 214788
Can be used this reaction for Mn(Ac)3:
4Mn(Ac)2 · 4H2O + KMnO4 + 8HAc > 5Mn(Ac)3 · 2H2O + KAc + 10H2O
Instead of boiling Mn(Ac)2 in GAA ?
Ref:
http://www.dissociative.h1.ru/chemic/otd
(Hive Bee)
09-18-01 12:12
No 214855
I'm trying to make a possible (TOTALLY theoretical) mechanism for this process. But I've one question:
The reaction?
(CH3COO)3Mn(III) + CH3COOH + C6H6 + CH3COCH3 -------> C6H5CH2COCH3 + (CH3COO)2Mn(II) + WHAT?
I've lost a couple of protons....
Can you help me? Thanks
If Rhodium can help me, I'll get my response on his e-mail (or any anonymous else), cause I've to make some images but I don't have any http to get them.
(Hive Bee)
09-18-01 13:36
No 214877
Ehm.... another question:
There is a production of H2?
(Chief Bee)
09-18-01 14:28
No 214899
I haven't got any email from you, try rhodium@privacyx.com - my hushmail is malfunctioning.
(Hive Bee)
09-19-01 01:51
No 215077
Ops, I'm stupid.... THIS IS the reaction involved...
Sorry!
(Chief Bee)
09-19-01 03:06
No 215090
Taken from Fester's 5th Ed of his book:
To get Mn(III) acetate from Mn(II) acetate, we return to a recurring theme in industrial chemistry - the electric generation of Mn(III) from Mn(II). We saw one example of this kind of conversion back in Chapter Nine in the benzaldehyde recipe. For this next one see Acta Chemica Scandinavica B 33 (1979), pages 208-212. At a graphite or platinum anode in a simple, undivided cell, using a cathode much smaller than the anode to minimize reduction of the Mn(III) formed, the chemists produce Mn(III) acetate from Mn(II) in glacial acetic acid solvent.
One mole of Mn(II) acetate is dissolved in one liter of glacial acetic acid. A little bit of sodium lithium fluoroborate (a few grams) is added as current carrier to the solution. One could also try sodium or potassium acetate as current carrier; it may not interfere in this reaction. The fairly large graphite or platinum anode is placed in the solution, along with the smaller cathode. The mixture is warned, and then with stirring, DC current is made to flow through the cell. One should apply 4 milliamps of DC current for each square cm of anode surface facing the cathode. This is a one-electron oxidation, and one can count on getting around 66% efficiency in the oxidation. So one should pass about 1.5 faradays of current. One faraday is 96,500 amp seconds, so if for example one is passing one amp through the solution, the electrolysis to Mn(III) will take 40 hours. At four amps, it will take 10 hours, and so on.
At the end of the electrolysis, one has the Mn(III) acetate solution in acetic acid. Then to this solution, one can add the benzene and acetone with stirring, and react as usual. It's a lot of work to get 30 ml of phenylacetone, but those chemicals certainly are low profile, cheap, and easily available.
For another example of electric generation of Mn(III), see US Patent 4,560,775.
(Hive Bee)
09-19-01 06:03
No 215113
I've posted a possible mechanism to your email rhodium@privacyx.com, Chief.
(Chief Bee)
09-20-01 02:27
No 215495
That mechanism is way over my head, but you can read it at ../rhodium/archive/mechanism
(Hive Bee)
09-20-01 02:47
No 215500
Thanks Chief!!!
Ehm, but I'm a silly man... I've forgotten to sign that .doc!!!!
DarkStarCrashesPouringItsLightIntoAshes
(Newbee)
09-20-01 11:30
No 215659
The Mn(Ac)3 synth seems to work just fine, will post yields after crystals have finished falling out.
I didn't leave out the step of evapping the GAA first and then redisolving Mn(Ac)2 back in. It does take a 2 hour reflux to get it to dissolve.
The Mn(CO3)2 that I got was not white though, it was a very light pink color. (Which is probably why - in the original post - the solution after dissolving it in GAA is a "pleasant pink".
Who knows, I may have fucked up, but the rest of the reaction colors seemed exactly as described. Is it possible that I made something else?
(Chief Bee)
09-20-01 13:58
No 215694
I am interested in the preparation of Manganese(III)acetate.
We know from experimental evidence that the following reaction can occur:
KMnO4 + HCl + C6H12N4 => MnCl2 + KCl + ?
So the reaction could be something like that the hexamine is being hydrolyzed by hydrochloric acid to ammonium chloride and formaldehyde, the latter is then being oxidized by the potassium permanganate to formic acid like this:
2 KMnO4 5 HCHO 6 HCl => 2 MnCl2 2 KCl 5 HCOOH 3 H2O.
Or perhaps the formaldehyde gets oxidized all the way to carbon dioxide, like this:
4 KMnO4 5 HCHO 12 HCl => 4 MnCl2 4 KCl 5 CO2 16 H2O
Why is hexamine used, and not plain formaldehyde? Is the ammonium ions there to act as a pH buffer, or is is just convenient to use the less toxic hexamine? What is the reference for this reaction?
Anyway, the following reaction can occur, and could be another way of making MnCl2 (the MnO2 can be had from batteries):
MnO2 4 HCl => Cl2 MnCl2 2 H2O (this is how Scheele discovered chlorine in 1774)
The precipitation of manganese(II)carbonate is trivial:
MnCl2 2 NaHCO3 => 2 NaCl MnCO3 CO2 or MnCl2 Na2CO3 => 2 NaCl MnCO3
and its conversion to the manganese(II)acetate is too:
MnCO3 2 AcOH => Mn(OAc)2 CO2 H2O
But, how does the KMnO4 oxidation of manganese(II)acetate proceed? Have we references for this?
A very plausible reaction is 4 Mn(OAc)2 5 HOAc KMnO4 => 4 Mn(OAc)3 KOAc MnO2 according to experimental observations.
(Chief Bee)
09-20-01 15:09
No 215706
What about the following mechanism for the coupling between acetone and benzene:
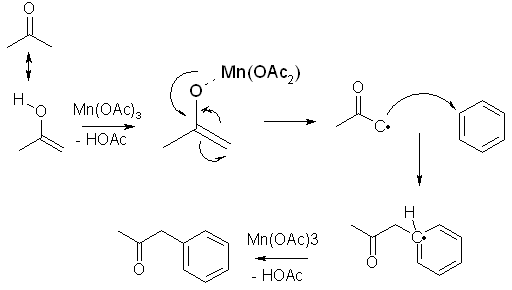
This explains the low yield (40%) based on the number of moles of Mn(III) used. One equivalent of Mn(III) is needed to first generate the free radical, and one equivalent is needed to terminate the radical reaction. No hydrogen are lost in this mechanism, and it explains the low molar yield, which is actually an 80% chemical yield, but it is halved because two equivalents are needed for the reaction.
Reference for the above: http://www.york.ac.uk/depts/chem/staff/a
These articles might also be of interest (haven't checked them out yet):
Linker, U.; Kersten, B.; Linker, T. "Potassium Permanganate-Mediated Radical Reactions: Chemoselective Addition of Acetone to Olefins" Tetrahedron 1995, 51, 9917-9926.
Linker, T. "Manganese(III)-acetate – A Versatile Reagent for Radical Generation and C–C Bond Formation" J. Prakt. Chem. 1997, 339, 488-492.
I find it amusing that a guy called "Linker" has written an article on "C-C bond formation"
(Hive Addict)
09-20-01 16:26
No 215728
nice mech proposal- both Rhodium and Peyote. and Rhodium- you are too easily amused, you'd think that with your apparent intelligence you could find something a little more in depth to amuse yourself. but hey, simply pleasures for simple people
(Chief Bee)
09-20-01 17:36
No 215747
Life would be very boring if one wouldn't be able to find some level of amusement in even the dullest of places (like in a list of references).
(Hive Addict)
09-20-01 19:18
No 215779
don't get obit wrong here- obit was also amused. like obit said simple pleasures for simple people. obit just didn't state obit as being one before now.
(Hive Bee)
09-21-01 02:21
No 215879
Referring to:
Is the ammonium ions there to act as a pH buffer, or is is just convenient to use the less toxic hexamine?
May the NH3 work as ligand?
Just A Touch Of Mojo Hand
(Hive Bee)
09-21-01 02:25
No 215881
Hey Chief, correct me If I wrong, but there is a 5 bonded carbon in your picture... is it an intermediate like a SN2 mechanism? I hope this.
However your mechanism is very very very interesting
Just A Touch Of Mojo Hand
(Hive Bee)
09-21-01 02:29
No 215882
Thanks obit, but I wasn't only amused, I was so curious, interested and in a complete creative ecstasy (not MDMA,
Just A Touch Of Mojo Hand
(Chief Bee)
09-21-01 02:53
No 215885
Yes, there is a 5-valence carbon drawn in the intermediate formed after the acetone radical has added to the benzene ring. The radical is in reality distributed throughout the aromatic ring though, thereby making it more stable.
I believe someone should try to perform the reduction of KMnO4 with only formaldehyde and see what the results are (like if 1.25 or 2.5 molar equivalents of formaldehyde is needed) and if more acid is required than indicated in the equations above, or if that is just enough.
(Ubiquitous Precursor Medal Winner)
09-21-01 05:51
No 215918
Did I miss something? When Antoncho said "camping fuel (SWIM used hexamine cause he had it at hand - any ammonium salt will do just fine)" I was thinking ammonium sulfate (or ammonium nitrate, which I was very hesitant to try
turning science fact into <<science fiction>>
(Official Hive Translator)
09-21-01 06:09
No 215922
So do i
You can reduce KMnO4 to Mn(II) w/just about anything, as long as pH is acidic.
Antoncho
(Chief Bee)
09-21-01 08:03
No 215949
Yes, but what was the rationale for the hexamine? And is the equation for the KMnO4 oxidation of Mn(II) to Mn(III) correct?
(Newbee)
09-21-01 12:10
No 216007
Rhodium:
I am interested in the convertion as well. Swil is in a posistion right now to do a few experiments with about a Kilo of Manganese(II)chloride anhydrous MnCl2 in hand and willing to try anything. What shall we try first?
Rdy2go
...
..
.
Hum did you get that?
(Chief Bee)
09-21-01 14:24
No 216044
Precipitate the MnCO3 with carbonate, dry it and record the yield. See if it is quantitative.
Make the Mn(OAc)2, dry, and record the yield.
Make the Mn(OAc)3, and record the yield of both the formed triacetate and the brown precipitated MnO2, so we can see if the reaction needs tweaking.
(Hive Bee)
09-27-01 00:46
No 217631
Hey, see my Post 214788 (PEYOTE: "Re: P2Ps from benzene, acetone and Mn(AcO)3", Novel Discourse), some post ago.
Or visit:
http://www.dissociative.h1.ru/chemic/otd
I've written these things some time ago.
(Hive Bee)
09-27-01 03:54
No 217669
Another way from Mn(II)-acetate to waterfree Mn(III)-acetate is to dissolve the Mn(II)-acetate in Acetanhydrid (AA) which is unfortunately even more difficult to get than Kaliumpermanganat!
Carpe Diem
(Newbee)
09-28-01 13:25
No 218216
could you please post this reference. I canot read it?
Thanks
Hum did you get that?
(Hive Bee)
09-29-01 08:36
No 218418
Check USP 4,011,239
they say Mn(III)acetate is made by dissolving any manganese oxide in acetic acid.
e109
(Master Searcher)
09-29-01 09:50
No 218426
In US patent 4,011,239 see column 20 (page 11 of the images) last paragraph (lines 43+), column 21 (page 12) down to about line 32 and column 23. A lot of useful info in the patent.
In column 23 it also says
Alternatively, the isolated manganous acetate may be dissolved in acetic acid and the solution electrolyzed, using a carbon or other suitable anode, to form manganic acetate, the resulting electrolyzed solution being directly usable in a free radical reaction. Where the manganous acetate is already in solution in acetic acid, no preliminary isolation step is necessary as such solution may be charged to the electrolysis cell and electrolyzed.
http://www.geocities.com/dritte123/PSPF.
(Hive Addict)
09-29-01 13:36
No 218469
does anybody here a suggested procedure to run? this is very interesting and if at all possible obit would like to try running some experimental trials.
(Chief Bee)
09-29-01 13:56
No 218473
Obit: What more do you want? The preparation of Mn(III)acetate seems pretty straightforward, and so does the condensation.
(Hive Addict)
09-29-01 17:46
No 218552
sorry- missed the first portion (forgot about the first part of the thread)
sincere apologies
suggestions on anything are still welcome though.
(Hive Bee)
09-30-01 11:47
No 218790
Hi guyz. I've mumbled and mumbled and I think.... referring to:
It works even better w/substituted benzenes: for anisole the yield is 74% (in 45 min), unfortunately a mixture of isomers is formed of which ortho- predominates. Para-dimethoxybenzene etc etc etc
on Antoncho Post 205055 (Antoncho: "P2Ps from benzene, acetone and Mn(AcO)3", Novel Discourse).....
.... if I use an aniline or an N-monosubstituted aniline (also this possibly substituted) instead of benzene, is it possible to make a substituted indole, and open a way for new DMT and related synthesis?
I'll email a possible explanation to Antoncho, if he'll want.
(Hive Bee)
09-30-01 13:15
No 218816
I was thinking, suppose someone has 20 grams of 2,5-dimethoxybenzene, obviously the best choice here (yield-wise) and is willing to do an experiment.
Is there anyone that has a clue on the total yield here, because i don't seem to understand. If 20gr will give only a sub-gram amount of the corresponding P2P i don't think it's worth the effort.
What's the best we can do here ?
e109
(Hive Bee)
09-30-01 13:22
No 218819
suppose someone has 20 grams of 2,5-dimethoxybenzene
Ehm, 2,5-dimethoxybenzene? How can someone have 20 g of 2,5-dimethoxybenzene? Ah.... 20 g of 1,4-dimethoxybenzene, or para-dimethoxybenzene !
However, the TOTAL THEORETICAL POSSIBLE YIELD from 20 grams of 1,4-diMeO-Bz to 2,5-dimethoxy-P2P is about 28,12 grams
Just A Toch Of Mojo Hand
(Hive Addict)
09-30-01 14:32
No 218841
is the product worth tasting? (after amination obviously)
and what solvent would be best? just straight acetone in a large excess?
(Chief Bee)
09-30-01 14:54
No 218846
2,5-dimethoxyamphetamine is not good in itself, but it can be brominated to give DOB for example (and chlorinated, iodinated, nitrated etc).
(Hive Bee)
10-04-01 04:00
No 220197
Extracts from JACS, 51, (1969), 143-144
Procedure #1:
In a 5-l., 4-necked round-bottom flask fitted with a stirrer, condenser and a thermometer, 428.7 g of Mn(OAc)2 . 4H2O in 3 l of GAA was heated to 110°C. Ground KMnO4 (68.2 g) was added in small portions through the condenser over a 20 min. period, while the temperature was mantained at 110°C. The rxn mixture was heated an additional 20 min., cooled, poured into water (750 mL), and left to crystallize overnight. The solid was filtered off, washed with ether and air dried. Yield: 486 g (82%)
Procedure #2: (FOR THE ANHYDROUS PRODUCT)
A mixture of Mn(NO3)2 . 6H2O (60 g) and Ac2O (225 mL) was stirred vigorously in a 4-l. beaker and heated cautiously until the cloudy mixture suddenly turned clear. The heating was stopped and a vigorous rxn followed during which there was a copious evolution of NO2. The product was allowed to crystallize overnight, filtered, washed with Ac2O, and dried over P2O5. The Mn(OAc)3 anhydrous thus obtained titrated as 90-98% Mn(OAc)3. Yield: 70-90%.
(Hive Bee)
10-07-01 05:57
No 221229
I'm sorry... of course it's 1,4-dimethoxybenzene.
Then how comes one gets only 134mgs of P2P from 15mls of benzene and calls it 40% yield. I don't seem to understand this.
e109
(Chief Bee)
10-07-01 07:28
No 221241
They calculate the yield on the basis of added Mn(III)acetate, and not on the added amount of acetone and benzene.
(Official Hive Translator)
10-07-01 07:52
No 221247
Note too that two molecules of the Mn comp'd are needed to make one P2P molecule. Also note that Mn(AcO)3 molecule is almost two times heavier than P2P.
That's why
Now an idea/a question for everybody interested.
BTW, in one of the patents dedicated to this issue this salt was prep'd in situ. However, Ac2O was then used to dehydrate the rxn mixtr - it was stated that for the condensation the conditions must bee unhydrous, and water is obviously a product of KMnO4 rxn w/Mn(AcO)2/AcOH. So it is pretty seductive to suggest that maybee some more common dehydrating agent like Na2SO4 could bee used instead, and a pretty concentrated solution of MnAcO3 could bee thus obtained through a series of MnAcO2/KMnO4 rxns followed each time by dehydration and filtering (MnAcO3 is hydrolized by water, so you can't just do a bunch at a time).
For this - just to bee sure - we would only need to know how much Mn triacetate can bee dissolved in a given quantity of GAA.
Can anyone find this out? I know a couple of bees here who have access to real good chem. libraries
Antoncho
(Hive Bee)
10-08-01 09:26
No 221686
Thanks, i do understand now.
Well, i've been to the library to look up those "Linker" ref's from up above.
They don't have the J.Prakt.Chem. article from 1997, and Tet.Lett. 51,9917-26 (1995) doesn't exist, the pages only go to p.9566 .
I found in CA 77,151620 (1972):
CH3COCH3 and Mn(OAc)3 gives radical CH3COCH2* ; and this can add itself to C6H6. So, in AcOH at 70° there was formed 36% CH3COCH2Ph. Toluene reacted similarly and gave 30% tolylacetone and 7% PhCH2OAc. RR1C*COR radicals with R=alkyl and R1=H or alkyl did not add to C6H5 however, but recombined with themselves.
Anhydrous reagens and solubility apart, I understand that 7.8 grams of benzene, 53.6 grams of Mn(OAc)3 and 5.8 gr of CH3COCH3 reacts at 70° to give ~5.4 grams of P2P.
This all should be done in glacial AcOH as a solvent
One more thingie: tolylacetone is PhCH2CH2COCH3, right, isn't the amination product known to be active ?
e109
(Hive Bee)
10-08-01 09:52
No 221694
Yes, on JACS 1971, 93, 524 the Authors use an alkene (should be 1-undecene) and compare the ratios of oxidation rxn using Mn(III), Ce(IV) and Cu(II) acetate.
(Chief Bee)
10-08-01 11:39
No 221734
element 109: The correct ref is Tetrahedron, vol 51, no 36, p 9917-9926 (1995).
(Hive Bee)
10-11-01 08:43
No 223203
Chief, I'm very sorry, it was my mistake, i didn't know there was a difference between Tetrahedron and Tetrahedron Letters
Peyote: I've read that one too, wasn't the use of Cu(II) impractical because "the reversible interaction of Cu(II) with the radicals and thus represent a minimum value" (p.526,table I,note d)
It seems that SWIM is going to methylate 0.1Mol hydroquinone tomorrow, so we can see how promising this reaction is. He only needs to get ahold of 53.6gr of Mn(OAc)3, tho.
I wonder if the inert atmosphere is really needed in this rxn, the authors don't always use it.
e109
(Hive Bee)
10-15-01 01:26
No 224700
In the interest of those who don't have piles of
KMnO4 lying about, it might be of interest that
Manganese Oxide is available in huuuge amounts very
cheap at pottery supplies (SWIM picked up 250g of
Nickel Carbonate recently for $6 - there was a sale on).
Also, I expect many bees don't have too much carcinogenic
compounds (benzene) at their direct disposal, however
this could be easily synthesised from many other compounds.
I'll have a look into the reaction products of using
naphthalene as a substitution for benzene. This could
be useful for many people here, as a totally OTC synthesis
of P2P would be very interesting to say the least.
Don't mind me. I'm mentally ill.
(Stranger)
10-31-01 14:08
No 231119
I`m a litle confused now. In the begining of the discusion it was claimed that the reaction would give 40% yeld wich would be somthing like 6 ml P2P from 15 ml benzene, 15 ml acetone and 1g Mn(AcO)3. Then it was latere concluted that 7.8 grams of benzene, 53.6 grams of Mn(OAc)3 and 5.8 gr of CH3COCH3 would give ~5.4 grams of P2P wich is about a 100 times as much Mn(AcO)3 as claimed in the begining. You can just tell me that it is still the firs ratios that is the right ones, anything else would be TO disapointing.
(Official Hive Translator)
10-31-01 22:04
No 231431
A Golden Rule #2 (#1 is UTFSE) - always read the whole thread bee4 posting anything into it!
Thus you can avoid being flamed.
You are currently being flamed.
Antoncho
(Newbee)
11-22-01 07:08
No 239373
Yes it is unbeleveabull shit hard to get benzene. What else may be used in it's place. Has anyone come up with an optimiced ratio yet? How are things going? I have had some success going from MnCL2 to MnAco3 with NA carbonate then acetic anhydred. Watch out it liks to foam up alot and for quite a while...
Hum did you get that?
(Hive Bee)
11-24-01 07:04
No 239992
You can distill benzene from a 1:1 mixture of benzoic acid and calcium benzoate, if i remember correctly.
SWIM don't want no stinking benzene, he's got some p-dimethoxybenzene instead
e109
(Ubiquitous Precursor Medal Winner)
03-08-02 01:10
No 278841
The most direct and straightforward way to produce manganous acetate, is by the reduction (that's what it says here, folks) of KMnO4 with hydrogen peroxide in acetic acid. Reduction of potassium permanganate with hydrogen peroxide, except in sulfuric acid producing manganous sulfate, was given in one of my old textbooks as an example of the reducing ability of H2O2.
Well, I had no H2O2 at hand, so I used sodium perborate instead. I suspect any of the peroxide-generating oxidants, oxone or peroxicarbonate would serve as well, as of course would barium or sodium peroxide. I had fun, alternately turning acetic acid dark and clear, "black" and "white", by adding KMnO4 and NaBO3 respectively. This trial was just beecause I wanted to see it, but a little aliquot to which I added benzene and acetone seemed to do something to them, even without heating. No color showed up I would call "pink", but perhaps the manganic acetate only shows up with hot permanganate oxidation.
To prove I had manganous acetate, I titrated to basic with sodium carbonate, whereupon the carbonate precipitated, as in the manganese (III) acetate document at Rhodium's ../rhodium /mangano
a half a pints a half a pound a half a world a half a round
Sidearm n. Flask neck tube.
(Chief Bee)
03-08-02 04:12
No 278919
It sounds good - now if someone could just find a nice reference for that reaction with a suggested procedure, or that somebody would work out an optimized procedure from scratch...
(Distinctive Doe)
03-08-02 05:42
No 278936
Hmmm
Manganous formate and acetate.
Paltin, Edith; Grosu, Luminita; Selegeanu, Dinu.
RO 51886 19690924 Patent written in Romanian.
Abstract
The title compds. are prepd. directly from ores, which are treated with so lns. of org. acids in presence of a liq. oxidizer. Thus, to 100 kg 4.44% soln. of HCO2H, 4 kg 33% perhydrol was added, re sulting a 1.3% H2O2 content. This soln. was slowly added with stirring to 4 kg MnO2. Oxygen evolution ended after 1 hr at 20° and the mixt. was worked up to give 95 - 99.9% pure (HCO2)2Mn crystals. Similarly prepd. was Mn(OAc)2.
Refining of manganese acetate and cobalt acetate.
Haas, Zdenek; Foniok, Alfred.
Czech. (1974),CS 153278 19740515 Patent written in Czech.
Abstract
Cryst. Mn(AcO)2 and Co(AcO)2 were dissolved at 70° in 50% aq. EtOH contg. 7-10% HONH3OAc or (HONH3)2SO4 (on the wt. of alc.) and the soln. cooled to give 72-5% cryst. product in which the amt. of insol. material has dropped from 0.1 to 0.005% and the Fe content from 0.005 to 0.001%.
Crystalline manganese acetate.
Haas, Zdenek; Alfred, Foniok.
Czech.(1974),CS 152911 19740415 Patent written in Czech.
Abstract
Cryst. Mn(OAc)2 [638-38-0], useful as a highly active catalyst in polyester manuf., was prepd. by treating MnCO3 soln. with AcOH at 90° in the presence of a reducing agent, hydroxylamine sulfate [13973-61-0].
Study of the manganese acetate formation reaction.
Novak, A. M.; Abadzheva, R. N.; Bel'ferman, A. L.
Zh. Prikl. Khim. (Leningrad) (1977), 50(9), 2135.
Abstract
Mn(OAc)2 was prepd. by treating MnCO3 with HOAc in H2O in 1:5.5:36.0 ratio at 80° with high-speed stirring.
Make a Difference Donate Now!! (http://www.lp.org/drugwar/)
(Chief Bee)
03-08-02 11:46
No 279004
Foxy: Is it only people who speak incomprehensible languages who write articles/patents on the preparation of useful chemicals? I have seen that phenomenon over and over again.
(Master Searcher)
03-08-02 17:59
No 279190
One problem with US patents is that the attorney/patent agent writes them (they're applications first). They typically have a degree in life sciences or other science (ie. computer science) and/or engineering and may also have a law degree. They want to claim as much as possible and to do that the disclosure (the specification and drawings if any) has to be enabling (explains how to make and use the invention) therefore they make the specification as broad as possible which often makes the patent vague to some extent. This seems to be more true with more recent US patents. The more you read US patents the easier it gets to read them. BTW, any employee of the USPTO cannot write patent applications or do any patent work other than what they do in the office. Examiners can do amendments, but need the permission of the attorney/agent to enter them.
http://www.geocities.com/dritte123/PSPF.
(Ubiquitous Precursor Medal Winner)
03-08-02 22:57
No 279305
Thread's too long. Starting a new one, to bee called "Acetate of Manganese", Post 279306 (halfapint: "Acetate of Manganese", Novel Discourse).
a half a pints a half a pound a half a world a half a round
Sidearm n. Flask neck tube.
(Master Searcher)
03-30-02 21:51
No 290192
I didn't find these two UingTFSE.
Patent US3845116
Patent US3875224
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious