02-10-02 13:39
No 267698
Oh my beloved ones look what I have found. If you don’t immediately say it’s beautiful I’m gonna go hang myself.
IMO, it is so heavenly that I cry every time I think of it - Jesus F. Christ, could it bee so simple! <sniff>
From Patent GB1557237:
May I also humbly note that this is acceptable in case w/EtOH, although unoptimized (yield in the patent’s only ex. is 40%), leading us to exciting 5-Tweetios.
I also bet that, just like vanillin, 2-hydroxy-5-MeO-BA can bee methylated w/NaMeSO4 - but SWIM will have to get to that stage 1st/yet, well this is offtopic anyway.
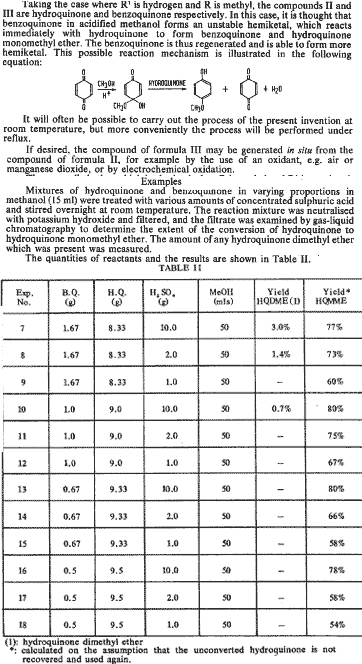
EDIT:In reality, neither of the too can bee methylated w/NaMeSO4 in a decent yield - SWIM was too naive back then
Antoncho
(Hive Prodigy)
02-10-02 13:46
No 267699
Could it be used for alkylated quinones?
Say for 2-ethylamino-1,4-benzoquinone?
Then only one half of the methylating agent is needed to methylate the remaining hydroxy.
PrimoPyro
Vivent Longtemps la Ruche!
(Official Hive Translator)
02-10-02 14:19
No 267703
Well not really...
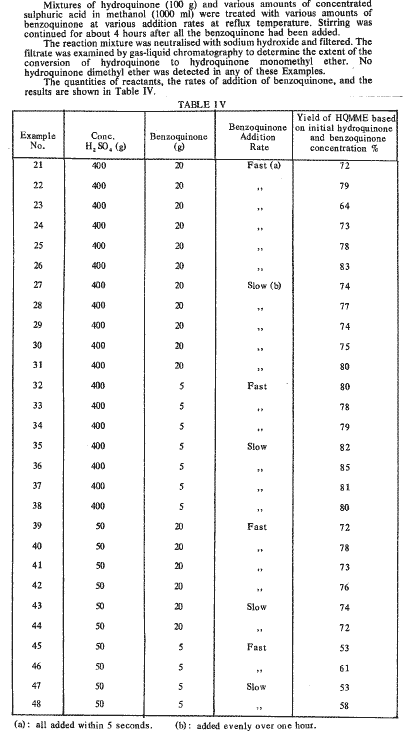
i think, if the mechanism the authors suggest is correct (and it does make sense, IMHO), then it'll work for any hydroquinone just fine.
But the alkylated species here is the hydroquinone, not the quinone, so i don't quite get the reasoning... maybee interesting 1st to reduce , then alkylate, then alkylate again using NaMeSO4 - i suppose, it doesn't alkylate amines, see what i'm aiming at?
The main point of my joy here - i feel the need to explain - is that p-methoxyphenol, and only p-methoxyphenol, is subject to Reimer-Tiemann formylation, and in good ~70% yield (tested by Karl) - which is very-very OTC, non-toxic - well, it's kitchen chemistry at its best.
The alternative (starting w/p-diMeO-benzene, which SWIM has successfully made w/NaMeSO4) is Vilsmeier formylation, which requires smth like POCl3 (unaffordable for SWIM) or SOCl2 (can bee made from SCl2, but in a VERY disgusting, stinky way). We'll just leave phosgene alone
The only previously known method of making p-methoxyphenol (BTW, also found by moi
An attempt to pull this rxn w/NaMeSO4 was made, but a major problem was encountered: toluene forms a low-boiling azeotrope w/water, and the temp. is insufficient for the NaMeSO4 methylation to effectively take place (although some product still has been isolated). SWIM was planning to try repeating it w/xylene, but w/out much hope, since bp of the azeotrope would bee lower than 100 C anyway.
Now having this procedure in hand... SWIM guesses it's time for him to start saving money for a bottle of chloroform, heh
Antoncho
(Hive Prodigy)
02-10-02 14:46
No 267704
Antoncho:
i think, if the mechanism the authors suggest is correct (and it does make sense, IMHO), then it'll work for any hydroquinone just fine.
Yes, I needn't be taught the difference. Perhaps I was unclear. This is what I meant. Reduction of the formed alkylated quinone to the hydroquinone, followed by alkylation of the hydroxyl group.
But the alkylated species here is the hydroquinone, not the quinone, so i don't quite get the reasoning...
Yes, that is precisely why it is so beautiful. The benzoquinone acts as sort of a catalyst. I also wonder if more benzoquinone and methanol, with longer reaction times, will result in much higher dimethylated hydroquinone? That would be a wonderful OTC methylation procedure.
maybee interesting 1st to reduce , then alkylate, then alkylate again using NaMeSO4 - i suppose, it doesn't alkylate amines, see what i'm aiming at?
Yes, I see your plan. All of the ideas are interesting to me.
The main point of my joy here - i feel the need to explain - is that p-methoxyphenol, and only p-methoxyphenol, is subject to Reimer-Tiemann formylation, and in good ~70% yield (tested by Karl) - which is very-very OTC, non-toxic - well, it's kitchen chemistry at its best.
Now this reaction I had not yet thought of. Good idea.
I was merely likening this procedure to being also applicable to the quinone alkylation for introducing the ethylamino group for the very novel production of 2C-B. The main issue with that thread, which I understand is not the topic of this discussion, was the final reductive alkylation of the carbonyl groups, whit the issue of protecting the amino group. When I read this, the first thing that I thought of was an easy way to alkylate without protecting the amine. Perhaps you can see my reasoning now.
Good find, Antoncho. I look forward to news of your progress.
PrimoPyro
Vivent Longtemps la Ruche!
(Newbee)
02-10-02 16:27
No 267716
Thanx Antoncho, it's very cool. But I'm sad there is only a few % of dimethylether, and tweetios made that way with 2-ethylamino-1,4-benzoquinone would be a mixture of 2 and 5 isomers. Anyway p-methoxy phenol is a most wanted chemical too. For me it is more interesting that 1,4dimethoxy for manuf of 2,5dmb.
"then alkylate again using NaMeSO4 - i suppose, it doesn't alkylate amines, see what i'm aiming at?"
i hope it doesn't alkylate amines, but I don't think so. Some research to be done.
(Hive Addict)
02-10-02 17:05
No 267730
This is cool. Apart from the reduction step, this smells like an almost-OTC way to 2CB.
(Official Hive Translator)
02-10-02 18:07
No 267746
Yes, yes, my dearest friends... I'm glad you appreciated it. This is precisely SWIM's goal - 2C-OTC.
If i ever do that, i'll ask my title to bee changed for that word
Antoncho
(Hive Bee)
02-10-02 20:59
No 267779
The only previously known method for making p-methoxyphenol? Oh my, this should have been posted a long time ago!
J. Chem. Soc 1926, pg. 393:
Quinol Monomethyl Ether.-The method of Ullmann (Annalen, 1903, 327, 116) for the semi-methylation of quinol was found to give poor yields and the following process was adopted after numerous trials. A solution of quinol (110 g.) in sodium hydroxide (100 g.) and water (700 c.c.), contained in a flask filled with hydrogen, was cooled to 12 degrees and vigorously shaken after the addition of neutral methyl sulphate (120 c.c.) in one portion. After about 5 minutes the mixture was cooled and the dimethyl ether collected (33 g., m.p. 56 degrees). The filtrate and washings were acidified with hydrochloric acid and cooled to about 8 degrees for about 1 hour, thereafter the monomethyl ether was collected, washed with ice water, and dried (45 g., m.p. 52-54 degrees). The aqueous solution was extracted with ether and the residue after evaporation of the solvent yielded to benzene a further 30 g. of less pure quinol monomethyl ether, m.p. 41-46 degrees. For our purposes, it was necessary to ensure the absence of quinol from the product. The material was dissolved in benzene and any quinol which crystallized was separated; the solution was then distilled and a product, m.p. 53-54 degrees, collected at 243-246 degrees. This was redissolved in benzene, and the solution repeatedly shaken with small quantities of cold water. The ether was then again distilled, b.p. 243-244 degrees, m.p. 56 degrees. No coloration was developed in alkaline solution in contact with air. The substance crystallised from light petroleum has m.p. 56 degrees, but when heated to about 200 degrees and quickly cooled, the m.p. is 53 degrees, changing in a week or two to 55 degrees. Crystals, m.p. 56 degrees, also change on keeping and the m.p. becomes 55 degrees. Although these changes are small, the phenomenon is a real one.
Yes, quinol is hydroquinone and neutral methyl sulphate is dimethyl sulphate. Probably there was no need to tell but at least for the sake of TFSE
(Hive Addict)
02-10-02 22:20
No 267794
So 4-hydroxyphenol to 4-methoxyphenol to 2-hydroxy-5-methoxybenzaldehyde to 2,5-dimethoxybenzaldehyde to the nitrostyrene to the phenylethylamine throw a 4-bromo group on... beautiful! Nice thinking. Best of luck with this procedure...
(Hive Addict)
02-10-02 23:50
No 267814
Moo: you can't beat methanol/sulfuric acid with respect to `OTC-ness'.
(Hive Bee)
02-11-02 01:00
No 267834
That is correct. I was also thinking that maybe some other methylation agents might be employed in the same manner... Ending up with mono- and dimethylated products is no problem as they can be separated easily.
(Official Hive Translator)
02-12-02 15:23
No 268566
Well, oh my beloved companions, oh my six-legged friends; the gimmick clearly works!
It is not complete, yet, but it clearly works.
Benzoquinone was obtained as in you-know-where/quinones.html. The points worth mentioning are :
1) The crystals were not yellow, but rather green (and a very urgent care was taken to wash the product from impurities w/ice-water)
2) The still-wet (it was figured, naturally, that the rxn was insensitive to water) 1,7 g quinone was used instead of 1 g dry quinone.
Thence were combined :
a) 50-60 ml MeOH
b) 10 ml H2SO4
The mixtr was brought to RT and in were tossed:
c) 10 g HQ,
d) 1,7 g wet BQ
Hydroquinone forms a cake. It took some shaking to get it all dissolved.
The mixtr is initially black and intransparent (quinhydrone formed), in a half-an-hour it is deeply brownish-red and clear. It was allowed to stand for 18 hrs at temp slightly elevated from normal.
Then the mixtr was alkalified and some preciptt formed, it was extrxt’d w/ toluene – no change in color or whatsoever, SWIM thinks next time he’ll just xtract it after diluting w/aqua.
Then again acidified, ex’d w/toluene, some NaHCO3 stirrred in, decanted, evap’d (SWIM did it on a electric oven/all that stuff, he has no idea how much p-MeO-phenol has evap’d w/toluene. There was some, even more than some
p-MeO-phenol is at this point a disgusting black oil, it doesn’t crystallize other that on good cooling. Fuck. No weight s presently available.
The next run is immediately being put into action, the improved results are soon to bee seen.
The main question – w/what does one recrystallize p-MeO-phenol? Hexane? SWIM has some.
Hope someone helps.
Antoncho
(Newbee)
02-12-02 22:18
No 268715
hi antoncho,
congratulations, this is good news. a very simple method for a good precursor. concerning clean-up, you could steam-distill the product, that should remove most impurities. at least the color will go.
otto
(Official Hive Translator)
02-13-02 01:06
No 268799
Aww!
Are you positive that it can bee steam-distilled? It's soluble in water like ~4 g/100 ml. But if you say it can, i'll gladly believe you.
And - does anyone by chance know if adding salt will help the steam-distillation - by lessening the solubility? Generally?
Eagerly awaiting your input, bees,
Antoncho
(Distinctive Doe)
02-13-02 04:43
No 268893
Antoncho
Check this out
US 4,469,897
Example #2
5.0 g of hydroquinone, 40 cm.sup.3 of methanol and 1.0 g of cupric chloride are fed into a teflon-lined autoclave having an internal capacity of 100 cm.sup.3. The autoclave is purged with nitrogen and is kept for 2.5 hours at 105.degree. C. On termination of the reaction, an analysis of the mixture indicates the formation of 4.1 g of hydroquinone monomethyl ether (conversion 85%, selectivity 87%).
Or how about this one.
US 4,294,991
Example 20
Hydroquinone (18 g) and benzoquinone (2 g) in methanol (100 ml) with p-toluene sulphonic acid (5 g) were heated under reflux for 6 hours. Samples taken at 3 and 6 hours were analyzed by gas-liquid chromatography and found to have the compositions indicated in Table III.
TABLE III
______________________________________
Hydroquinone
Monomethyl
Sample Benzoquinone
Ether Hydroquinone
______________________________________
After 3 hours
0.12 g 14.6 g 3.6 g
After 6 hours
0.00 g 15.8 g 2.8 g
______________________________________
Stay Informed (http://www.mapinc.org/)
(Hive Addict)
02-13-02 04:46
No 268895
I'm positive that it can be steam distilled. GBL, a water miscible solvent can be steam distilled even though it's completely soluble in water. Don't try to recrystallize until you have crystals.
(snip - I had posted suggestions to clean up the mix, but it's basically the same you're doing - snip)
(Distinctive Doe)
02-13-02 04:59
No 268898
This might give you some extraction ideas.
Its another procedure
Production of monoalkyl ethers of dihydric phenols.
US 3274260
Abstract
The title compds. were prepd. by treating an unsubstituted dihydric phenol with an alkylating agent in the presence of an aq. alkali metal hydroxide in a 2-phase system at 65-100°. The alkali metal hydroxide was added gradually in the presence of a relatively large amt. of an immiscible solvent. The monoalkali salt of the dihydric phenol remained in the aq. phase while only a small amt. of the alkali salt of the monoalkyl ether of the dihydric phenol will exist in the mixt. Thus, to 600 g. hydroquinone, 4800 g. C6H6, and 300 g. H2O at reflux temp. (70-5°) was added simultaneously 480 g. 50% aq. NaOH and 756 g. Me2SO4 under anaerobic conditions to minimize oxidn. of the hydroquinone. After refluxing an addnl. hr. the mixt. was acidified to litmus with approx. 12 g. HOAc. The top oil layer was sepd. from the aq. phase, washed with 600 g. 5% aq. Na2SO4 and distd. After removal of the C6H6, a 122-g. head fraction, b20 109-40°, and 525 g. hydroxyanisole (I), b20 141°, were obtained. From the head fraction, a mixt. of hydroquinone di-Me ether (II) and I, was obtained 53 g. I by extn. with aq. NaOH followed by acidification. A total of 578.5 g. I was recovered (78% conversion of hydroquinone). From the non-alkali-sol. portion of the head fraction was obtained 64 g. II (7.7% conversion of hydroquinone). The combined aq layers were extd. with methyl isobutyl ketone. Evapn. of the solvent gave 52 g. of 96% pure hydroquinone. The yields based on converted hydroquinone were 84.5% I and 8.3% II or a yield ratio of monoether to diether of 10:1.
Stay Informed (http://www.mapinc.org/)
(Stoni's sexual toy)
02-13-02 12:39
No 269043
Acidifying a solution containing 480 g. 50% aq. NaOH with only 12g HOAc???
Strange.
I'm not fat just horizontally disproportionate.
(Hive Prodigy)
02-13-02 12:47
No 269048
Perhaps a typo? 512g HOAc maybe?
The Water Will Be Your Only Mirror
(Official Hive Translator)
02-14-02 17:00
No 269667
Look what's been happening lately.
SWIM combined in a 250 ml flask 55 ml MeOH, 11 ml 92% H2SO4, 10 g hydroquinone (HQ) and 1 g (rather dry this time) benzoquinone. Just as beefore, the soln turned 1st intransparent and black, then in a half-an-hour all completely clear & dark reddish-brown. All was left for 20 h at RT w/out any stirring or even occasional shaking.
After the passage of the said time, into the mixtr was added 100 ml sat'd NaCl soln. - turned milky-brownish; extracted w/50 + 30 ml toluene, all color completely gone into the organic layer.
The toluene was stirred w/some solid NaHCO3 until all acid neutralized, then distilled off at oil bath temp ~130 C (it wasn't dried - assumed water would bee removed azeotropically, advice here? was SWIM wrong? Guess he was.)
Now - note this - toluene wasn't removed completely. The contents of the flask were poured into a glazed metal plate put on a boling water bath, and placed under draught. It was fucken incubated until almost all smell of toluene was gone, but never got absolutely rid of it! The moral: use smth lower-boiling for the extract'n!! SWIM isn't happy w/his results using toluene, at all. Possibly could result in lower selectivity, but considering the product has to bee further purified anyway (it's dark brown in color), it proally doesn't matter.
The brown oil was cooled, scratched somewhat - all immediately crystallized, the crystals were much nicer that in the previous attempt (when they looked more like dirt), although still somewhat moist/damp - guess some residual toluene or water.
The weight of this crude product was 8,4 g.
SWIM is now looking for any suggestions for further purification of the p-MeO-phenol. He's gonna steam-distill it 1st, but to get rid of that toluene... It won't hurt the planned Reimer-Tiemann, but of course having nice crystalline product would bee just... nice
2 PrimoPyro: sorry, but i ran out of Internet - all can do now is to post this messg
Antoncho
P.S. Ya lyublyu Yul'ku!!! Everyone happy holiday!!!
(Moderator)
02-14-02 17:37
No 269677
SWIM is now looking for any suggestions for further purification of the p-MeO-phenol. He's gonna steam-distill it 1st, but to get rid of that toluene... It won't hurt the planned Reimer-Tiemann, but of course having nice crystalline product would bee just... nice
You're going to have to apply a vacuum to the product, a test tube warmed in a water bath and an aspirator would be one good method ![]()
(Stoni's sexual toy)
02-14-02 18:07
No 269684
Recrystallization.
I'm not fat just horizontally disproportionate.
(Official Hive Translator)
02-15-02 12:15
No 269948
Dear Lugh! Of course, to vac distill the product would bee an ideal option. Unfortunately, SWIM doesn't have a source of vacuum. In fact, he doesn't even have a source of running water in his "lab", so he uses two buckets to feed the condenser (a 10 liter bucket lasts about 1,5 hrs
And, dear Osmium, SWIM fears that recrystallization might bee difficult as the product is very dirty. He will definitely try it the next time, though. As for now...
<sigh> Well, guys, steam-distilling p-MeO-phenol sucks! It doesn't steam-distill, or smth weird as fuck is happening – even worse, SWIM is starting to doubt if the product he got is what he expects.
1st, the liquid that comes over is totally clear. It does have something in it that is salted out though.
Then, p-MeO-phenol is obviously not very soluble in even boiling water - is that correct? At least, at 70 ml sol'n's volume, most of the oil stays undissolved. Upon addition of more water, however, it mostly dissolves.
But the main confusement is the fact that even at 150 C bath temp the mixture boils not very intensely – and I always thought that steam-distillation was based on the principle that the comp'd's azeotrope w/water boils at lower temp, no? WTF?
Well, after 6 hrs distillation (~100 ml distillate collected) the mixtr was diluted to 150 ml, boiled somewhat and decanted several times to separate the undissolved oil. The water phase immediately on cooling turns milky. It turns clear upon addition of alkali.
Well both water phases were pooled and some brown oil separated upon add'n of salt. It has a different smell from what it previously was - hard to describe, grassy, remotely similar to that of dimethoxybenzene. The smell used to bee very slight, oily. Can someone give SWIM a hint at how pure p-MeO-phenol smells?
Thanks in advance,
Antoncho
(Stoni's sexual toy)
02-15-02 13:20
No 269962
You don't have to dissolve your crude oil in water to steam distill it. But you might have to distill quite a lot of water, since I doubt the distillate will contain more than 1-2% of your product. You need bigger glassware, it will take forever and a day at a 16g/hr distillation rate. You might be able to speed it up by adding some salt to the distillation flask, so that the boiling point of the water will be raised, but don't expect too much from this.
To isolate the product you have to extract the distillate and evap the solvent again.
I'm not fat just horizontally disproportionate.
(Stranger)
02-15-02 15:13
No 269984
Antoncho,
I can at least clarify a point on steam distillation:
It has nothing to do with azeotropes. It relies on the fact that two immiscible liquids will each exert their own partial pressure, pa and pb. The total vapor press. will be pt = pa + pb, and the mixture will boil when pt = 1 atm. If there was only a or b present, it would boil only when pt = pa or pb respectively. Therefore the presence of the 2nd substance allows the mixture to boil at a lower temp. than either of the pure substances. The problem is that the distillate composition is in proportion to the vapor press. of each. Therefore it's usually only useful if the 2nd substance is significantly volatile in that range.
Anyhow, that's my 2 cents worth for today.
(Official Hive Translator)
02-15-02 15:39
No 269993
Osmium - thanks very much for the clarification, now Osmium (or some other xperienced bee), bee so kind - can you give SWIM an advice on rextaliz'n. He has a very general idea on how it is done and never actually performed one
a) While this crap is still an oil, it can bee just mixed w/an unspecified amt of hexane. Will it crash out as crystals on cooling/scratching or is this improbable? How much hexane should bee used per gram of the stuff?
b) Approximately, what percentage of impurities can the procedure tolerate? I mean, not if the crystals won't bee pure enough, but if they form at all.
Therefore the presence of the 2nd substance allows the mixture to boil at a lower temp. than either of the pure substances.
Yep yep yep. This is called Raoul's law or smth, right?![]() i'm shy to show my ignorance, of course, but... well...
i'm shy to show my ignorance, of course, but... well...
Anyway, the bp of the mixtr was definitely higher than 100 C - well SWIM was stupid enough not to measure it.
That's very confusing to SWIM.
Antoncho
P.S. Karl was so kind to inform me that p-MeO-phenol "has a kinda pleasant sweet hospital disinfectant aroma" - that's quite consistent w/the results from steam-dist'n. Thanks Karl!
(Stoni's sexual toy)
02-15-02 18:04
No 270029
> a) While this crap is still an oil, it can bee just mixed
> w/an unspecified amt of hexane.
It's impossible to predict how much hexane or whatever solvent will produce crystals, sometimes you only need to wet the oily residue with a solvent, sometimes you have to produce a hot solution which needs to be cooled and scratched etc. to crystallize.
> Will it crash out as crystals on cooling/scratching or is
> this improbable? How much hexane should bee used per gram
> of the stuff?
Try the following:
Place approx. 1g or less of your oil into a test tube or small vial, mix/extract with about 1ml warm hexane or MeOH or EtOH. In case there are insolubles separate the solvent with a pre-warmed pipet into another vial and let cool. It doesn't matter at all if only part of the stuff dissolves in your solvent since you want to create a warm saturated solution which is then allowed to cool separately. If nothing precipitates put it into freezer. If there is oiling out then you need more solvent and a slower cooling rate. You can also try to crash out the product by adding a bad solvent (like hexane) to a concentrated solution in a good solvent. Or putting a layer of the bad solvent on top of the solution, not disturbing the mixture and letting them mix by diffusion only. This might take quite a while, but patience is the key to crystallize such substances.
I'm not fat just horizontally disproportionate.
(Chief Bee)
02-15-02 18:06
No 270031
Mix the oil with twice the amount of hexane, and see if it dissolves completely. If it does not, but forms two layers, heat up the mixture lightly with stirring (but not above 50 C, as 4-MeO-phenol melts at 52 C, and 1,4-dimethoxybenzene at 58 C), and see if the solution is completely homogenous. If not, continue to add hexane slowly until it is. Now let the solution cool slowly, first at room temperature, and if no crystals forms, continue to cool in the fridge (and in a really bad case, the freezer). Scrape the inside of the beaker with a glass rod from time to time if the crystal formation is sluggish.
(Official Hive Translator)
02-16-02 08:36
No 270331
Thank you very much, dear maitres, esp. Os!
I am very sincerely grateful. Esp-lly considering smth. like this should bee asked in Newbee Forum
SWIM will get back and do it ASAP.
Antoncho
(Distinctive Doe)
02-22-02 02:17
No 271692
Antoncho
I think that a nonpolar extraction should isolate the product from a basic solution. If it works out as your ref. states you won't have any dimethylether to worry about.
Read this patent US 3274260 and watch how they isolate the products.
http://patft.uspto.gov/netahtml/srchnum.
Foxy
"After refluxing an addnl. hr. the mixt. was acidified to litmus with approx. 12 g. HOAc. The top oil layer was sepd. from the aq. phase, washed with 600 g. 5% aq. Na2SO4 and distd. After removal of the C6H6, a 122-g. head fraction, b20 109-40°, and 525 g. hydroxyanisole (I), b20 141°, were obtained. From the head fraction, a mixt. of hydroquinone di-Me ether (II) and I, was obtained 53 g. I by extn. with aq. NaOH followed by acidification. A total of 578.5 g. I was recovered (78% conversion of hydroquinone). From the non-alkali-sol. portion of the head fraction was obtained 64 g. II (7.7% conversion of hydroquinone). The combined aq layers were extd. with methyl isobutyl ketone. Evapn. of the solvent gave 52 g. of 96% pure hydroquinone."
Stay Informed (http://www.mapinc.org/)
(Official Hive Translator)
02-22-02 06:12
No 271794
Yes, Foxy, thank you - but the problem isn't solved this way. The problem is the dirt. It insists on staying together w/the product, no matter in what phase and what form it is - at least, it isn't extracted from an alkaline aqueous medium w/toluene. Not in the least degree. SWIM admits he hasn't given this approach much work though - maybee using smth like ethylacetate would work. He finally managed to make some money recently (money, not honey
Also, that patent you mention is a DMS alkylation, which is, AFASWIMK, a much cleaner rxn. I've seen it bee4 - actually, posted an xtract from it some time ago
So far, all attempts at rextallization were futile
SWIM's also made an xperiment using HMeSO4 and thoroughly dried MeOH - like, the purpose was to run the rxn totally unhydrous. Interestingly, the black precipitate that initially forms in the rxn, wasn't completely dissolved even after 3h (usually gone in 30 min), and when it did, the rxn was significantly darker than under hydric conditions. He hasn't worked it up yet, but looks like it ain't an improvement of the method - he'll see yet what the yield will bee, though.
That's it for now,
Antoncho
P.S. I'm terribly glad the Hive is back online
(Newbee)
02-24-02 11:36
No 272836
hi antoncho,
excuse otto for sugesting steam-destillation. otto himself used it for methyleugenol where it worked great.
please dont flame for next sugestion. you could possibly do a regular destillation of p-MeO-phenole (after drying). i saw in literature on several occasions a boiling point of 263°C. somehow it must have been determined...
otto
(Hive Addict)
02-26-02 02:08
No 273507
On my ride home, I was thinking about a variety of synthesis, especially the idea of OTC 2,5-dmb. Let's say for clarity that the following chemicals have the numbers:
1 4-hydroxyphenol
2 4-methoxyphenol
3 2-hydroxy-5-methoxybenzaldehyde
4 2,5-dimethoxybenzaldehyde
5 2,5-dihydroxybenzaldehyde
The route you're currently thinking about is 1 to 2 to 3 to 4, but why not go 1 to 5 to 4?
Of course this isn't a new idea, it's been discussed on Rhodium by applying Patent US4755613 to hydroquinone... but hopefully this will serve to generate some new dicussion!
P.S. To PP, sorry... I just revised my post (I often do that after I reread it, heh) to include patent reference... I hope that puts my question into a better context.
(Hive Prodigy)
02-26-02 02:16
No 273512
How does one make the conversion from hydroquinone to 2,5-dihydroxybenzaldehyde? I would like to hear your proposal, and perhaps I know his reasoning for choosing otherwise.
PrimoPyro
The Water Will Be Your Only Mirror
(Official Hive Translator)
02-26-02 08:16
No 273654
The answer is very simple: 20% yield. Megamole posted a procedure - admittedly, using the old-fashioned Reimer-Tiemann, nor the modernized version, but still sucks, IMHO.
Isolation of the product tends to bee reallydifficult w/such yields, also the hentisylaldehyde thus obtained is quite unstable/air sensitive and is proally a bitch to work with.
That's why SWIM's plans came to bee as expressed above.
Antoncho
Edit: I'm positive now p-MeO-phenol can't bee steam-dist'd - i looked up, it's vapor pressure is 10 times lower than that of p-DMB and even lower than veratraldehyde's - the latter can't bee steam-dist'd - an established fact.
(Stoni's sexual toy)
02-26-02 11:41
No 273718
(Rated as: excellent)
There is an excellent procedure starting with hydroquinone or 4-MeO-phenol, using SnCl2 or SnCl4, an amine and paraformaldehyde in toluene solvent.
Patent US4151201
Patent US6080895
JCS Perkin I, 1980, 1862
Then there's another one using phenols, Mg(OMe)2, paraformaldehyde and toluene. 4-MeO-Phenol to the aldehyde in 92%.
JCS Perkin I, 1994, 1823-31
Acta Chem. Scand., 1999, 53, 263-68
I'm not fat just horizontally disproportionate.
(Distinctive Doe)
02-26-02 19:48
No 273859
(Rated as: excellent)
Nasreen, Aayesha; Adapa, Srinivas R.
Aromatic hydroxylation by a new cupric nitrate-H2O2-phosphate buffer system.
Org. Prep. Proced. Int. (2000), 32(4), 373-376.
Get this article and it has said transfomation in 90% yeild.
The reagents listed are H2O2, Cu(NO3)2, Na2HPO4, KH2PO4, Water and MeCN.
Looks interesting to Foxy
Can anyone dig up this reference?
Speak Up(Action Alerts!) (http://www.drcnet.org/)
(Hive Addict)
02-26-02 20:16
No 273870
Didn't megamole write this:
However, I do have some good news. I did a review of some literature on the Reimer-Tiemann reaction for you, and while the formylation of hydroquinone or 4-methoxyphenol is not exactly high-yielding, it is quite reasonable (60-70%.) Considering how cheap hydroquinone is, I think its worth it."
Then go onto say in Post 224056 (megamole: "Re: Vilsmeier mechanism: the alternatives to POCl3", Chemistry Discourse) that gentisinaldehyde (2,5-dihydroxybenzaldehyde) could be prepared in good yields? 100g CHCl3, 550ml aq. NaOH, 10g hydroquinone... then extract with NaHSO3, wash with ether, acidify, extract and get the crude product?
(Official Hive Translator)
02-27-02 07:42
No 274101
2 foxy: i've seen a bunch of patents like this - they all claim high yields - except the fact that the ratio of hydroxylated species to the reagent is like 10:1 - needless to say, they calculate the yields based on the converted phenol/anisole. And they determine yield by chromatography - imagine what bitch it would bee to isolate the product (in kitchen, anyways).
I'm not sure about this one, though - just mentioning this FYI.
2 Chromic: damn it, i recall Megamole told me that in a PM, but god damn it can't seem to fucken find it! I might bee hallucinating of course, so the question remains open. I sinseriously doubt though that HQ could bee R-T-formylated in high yield - it is xtremely sensitive and turns to tar under much milder conditions - believe me, SWIM's done many things to HQ. Of course, everything may happen, OTOH.
Now guys, SWIM's grown really desperate about recrystallization of the freaken p-MeO-ph - no doubt, someone w/a more adequate lab technique could've managed it, but as you all of course know, SWIM's practical experience in the field is approaching zero (actually, away from zero, so to say
So the last idea he came up with - since the bitch is so fond of oiling out even at low temps, why not try to recrystallize the phenolate? He sees it like: dissolve the crude xtracted product in minimal 20% NaOH, wash w/non-polar (actually, this proally can bee skipped - it pulls absolutely nothing from the soln, even EtOAc); then carefully evap water and dissolve in boiling MeOH, then chill slowly.
Can anyone help him w/this phenolate's solubility in MeOH - or, maybee, some other solvt or solvt system would bee better used?
Yours as ever,
Antoncho
(Distinctive Doe)
04-03-02 22:28
No 291934
(Rated as: excellent)
Shit I initially thought they did this in acetonitrile but the said transformation is in carbon disulfide.
New reagents 3: aluminium iodide - a highly regioselective ether-cleaving reagent with novel cleavage pattern
M. VivekanandaBhatt, J. RameshBabu
Tetrahedron Letters 25(32) 1984 pgs3497-3500
Preparation of AlI3:
Dry aluminium foil (250 mg, 9.3 mmol) and iodine (1.9 g, 15 mmol) were refluxed in dry Cs2 (10 ml) or dry CH3CN (8 ml) till the iodine color disappeared (~3 hr).
Cleavage of allyl phenyl ether in CH3CN:
To a freshly prepared solution of AlI3 (5 mmol) in CH3CN, allyl phenyl ether (670 mg, 5 mmol) in CH3CN was added and the concentration of the solution was adjusted to approx. 1M with respect to both reagent and reactant. The reaction mixture was refluxed till there was no more starting material (5 hr. TLC), cooled and poured into water. The mixture was extracted with ether and the aqueous extract was washed with 5% sodium hydroxide solution. After acidification of the alkaline aqueous solution, it was extracted into ether, dried over anhydrous MgS04 and the solvent was removed. The crude product after short path distillation yielded phenol; 418 mg (89%).
Cleavage of 1,3-benzodioxzole in CS2:
To a freshly prepared solution of A1I3 (10 mmol) in CS2, 1,3-benzodioxzole (610 mg, 5 mmol in CS2 (2 ml) was added and reflexed till there was no more starting material (7 hr. TLC). The cooled reaction mixture was decomposed with ice, extracted with ether and washed with thiosulphate solution. The thiosulphate solution was extracted once again with ether and the combined ether extract was dried over anhydrous MgSO4. The solvent was removed and the product was chromatographed (TLC silica gel; 3:l hexane:ethylacetate) to obtain catechol, 440 mg (80%); m.p. 106C
Other Demethylations
----------------------------------------
p-dimethoxybenzene to p-methoxyphenol
substrate:reagent ratio - 1:1
solvent - CS2
Reflux time (hours) - 3
Yeild (%) - 74.2
p-dimethoxybenzene to hydroquinone
substrate:reagent ratio - 2:1
solvent - CS2
Reflux time (hours) - 4
Yeild (%) - 85
Those who give up essential liberties for temporary safety deserve neither liberty nor safety
(Master Searcher)
06-06-02 01:38
No 318010
PUB-NO: DE003207937C1
DOCUMENT-IDENTIFIER: DE 3207937 C1
TITLE: Process for the preparation of 4-alkoxyphenols
PUBN-DATE: May 19, 1983
ASSIGNEE-INFORMATION:
APPL-NO: DE03207937
APPL-DATE: March 5, 1982
PRIORITY-DATA: DE03207937A (March 5, 1982)
INT-CL_(IPC): C07C041/09
EUR-CL (EPC): C07C041/09
US-CL-CURRENT: 568/650
ABSTRACT:
A novel process for the preparation of 4-alkoxyphenols by reacting hydroquinone with an alcohol at increased temperatures in the presence of catalytic amounts of benzoquinone and an acid is characterised in that perchloric acid is used as the acid. This process gives 4-alkoxyphenols in high yields. 4-Alkoxyphenols are valuable intermediates, for example for the preparation of 4-alkoxyphenyl
carboxylates, which are used as liquid-crystalline compounds.
Patent DE3207937
********************************
PUB-NO: EP000039484A1
DOCUMENT-IDENTIFIER: EP 39484 A1
TITLE: Process for the production of monoethers of hydroquinone and quinol ketales as intermediates thereof.
PUBN-DATE: November 11, 1981
INVENTOR-INFORMATION:
NAME COUNTRY
KELLER, REINHOLD DR N/A
KONZ, ELMAR DR N/A
ASSIGNEE-INFORMATION:
NAME COUNTRY
HOECHST AG DE
APPL-NO: EP81103259
APPL-DATE: April 30, 1981
PRIORITY-DATA: DE03017393A (May 7, 1980)
INT-CL_(IPC): C07C043/23; C07C043/295 ; C07C041/18 ; C07C043/315
EUR-CL (EPC): C07C043/23; C07C043/305, C07C043/315 , C07C045/51 , C07C049/753
, C07D317/64
US-CL-CURRENT: 568/650,568/652
ABSTRACT:
Monoethers of hydroquinone are prepared by reduction of monoketals of
p-benzoquinone, preferably using complex hydrides of boron or aluminium, with a sulphite or molecular hydrogen in the presence of a nickel catalyst until one mole of H2/mole of p-benzoquinone monoketal is absorbed in an inert solvent to give the corresponding quinol ketals and treatment of these with an acid. The quinol ketals formed as intermediates here are novel compounds.
The monoethers of hydroquinone are precursors, intermediates and final products in various subject areas.
Patent EP39484
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Master Searcher)
06-06-02 04:01
No 318063
I wonder how allyl alcohol would work in a reaction like this. You probably have to use something other than H2SO4 as the dehydrating agent. Do a Claisen rearrangement on the hydroquinone monoallyl ether to get 2,5-dihydroxy allylbenzene, methylate it to get 2,5-dimethoxy allylbenzene and go from there.
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Chief Bee)
12-30-03 23:03
No 479833
(Rated as: good read)
Study of the steam distillation of phenolic compounds using ultraviolet spectrometry
Norwitz, George; Nataro, Nicole; Keliher, Peter N.
Anal. Chem., 58(3), 639-41 (1986) (../rhodium/pdf /steam.distil
Abstract
The steam distn. of 42 phenolic compds. was studied using a semimicro steam-distn. app. and UV spectrometry. In the distn., the following gave >95% recoveries: PhOH, 2-RC6H4OH (R = O2N, MeO, Br, Cl), 2,3- and 2,4-Cl2C6H3OH, 2,4,5- and 2,4,6-Cl3C6H2OH, 2,4-Br2C6H3OH, 2-, 3- and 4-cresol, 4,2-Cl(Me)C6H3OH, 2,4-, 2,5-, 2,6-, 3,4- and 3,5-xylenol, 4-RCMe2C6H4OH (R = Me, Et), thymol and carvacrol. The percent recovery for the other phenolic compds. was as follows: 3-O2NC6H4OH, 3.7%; 4-O2NC6H4OH, 1.8%; 3-MeOC6H4OH, 31.1; 4-MeOC6H4OH, 23.2; 3-BrC6H4OH, 79.6; 4-BrC6H4OH, 67.8; 3-ClC6H4OH, 93.5; 4-ClC6H4OH, 91.6; 3,4-Cl2C6H3OH, 64.1; 2,4-(O2N)2C6H3OH, 21.2; picric acid, 0.0; 2-H2NC6H4OH, 0.1; 3-H2NC6H4OH, 0.2; 4-H2NC6H4OH, 0.1; pyrocatechol, 1.6; resorcinol, 0.4; hydroquinone (I), 0.0; pyrogallol, 0.7; and phloroglucinol, 0.1. Examg. the spectra of the undistd., distd. and residual solns. showed that the aminophenols undergo some decompn., and that I is almost completely destroyed during the distn. The important role that H bonding (intermol. and intramol.) plays in the recovery from steam distn. is examd.
The Hive - Clandestine Chemists Without Borders
(A Truly Remarkable HyperLab Bee)
07-02-04 17:23
No 516970
(Rated as: excellent)
O-Monoalkylation of hydroquinone with alcohols
A. G. Rybin, A. V. Orlov, E. N. Zil'berman, M. Z. Barskova
Zh. Org. Khim., 27(9), 1828-1831 (1991).
(journal written in Russian)

The article was requested by synthon. A major part of it is a kinetic study of the reaction of hydroquinone with primary aliphatic alcohols in the presence of quinhydrone and H2SO4. The method is based on Patent US4294991 (mentioned in Post 268893 (foxy2: "Re: P-MeO-phenol from hydroquinone: part II", Novel Discourse)). Here is the translation of the synthetic part:
4-Alkoxyphenols, ROC6H4OH.
a. (R = CH3, C3H7, C4H9, i-C4H9).
A thermostatted glass reactor equipped with a magnetic stirrer and a reflux condenser was loaded with hydroquinone (8 g), quinhydrone (3 g), an alcohol (ROH, 100 ml) and sulfuric acid (8 ml). The mixture was stirred at 70 °C for 20 min; the unreacted alcohol was evaporated; the residue was taken up in water (50 ml) and extracted with PhMe (2×50 ml). The combined extracts were fractionally distilled in a vacuum (5 mm Hg).
b. (R = C5H11, C6H13, C7H15). The reactants were mixed as above, and the mixture was brought to boiling until the reactants dissolved completely. Then it was cooled, washed with water to remove the acid, and fractionated in a vacuum.
All the phenols obtained were recrystallized from heptane.
| R | yield, % | m. p., °C |
| CH3 | 80 | 54-55 |
| C3H7 | 64.1 | 55-56 |
| C4H9 | 48.8 | 64-65 |
| i-C4H9 | 17.2 | 64-66 |
| C5H11 | 30.2 | 46-47 |
| C6H13 | 28.2 | 47-48 |
| C7H16 | 7.7 | 59-60 |
07-08-04 22:33
(Rated as: off-topic)