(Hive Bee)
02-16-03 21:21
No 408642
(Rated as: excellent)
Here’s that reference GC_MS was looking for on Friedel-Crafts acylation using only Zn metal as a catalyst, I finally found it in my library (thanks Chimimanie
ZINC PROMOTED REGIOSELECTIVE FRIEDEL-CRAFTS ACYLATION OF ELECTRON RICH ARENES
Synthetic Communications 28 (12), 2203–2206 (1998)
H.M. Meshram, Gondi Sudershan Reddy, M. Muralidhar Reddy and J.S. Yadav
Indian Institute of Chemical Technology, Hyderabad-500 007, India
Abstract: A simple and convenient zinc promoted selective Friedel-Crafts acylation is described. The inexpensive metal grade catalysis and minimum waste effluent are the important features of the procedure.
The general experimental is as follows: A solution of acid chloride (4 mmol in 50 mL toluene) was stirred with activated zinc (4 mmol) at RT for 15 minutes. To this was added a toluene solution of arene (4 mmol in 30 mL) and the mixture was heated to 70oC for stipulated time (see table). The completion of the reaction was followed by TLC. The used up catalyst was removed by filtration, filtrate washed with sodium bicarbonate (10%) and organic phase dried over sodium sulfate. The evaporation of solvent gave essentially pure product in good yield. If necessary the further purification can be done by passing through a small bed of neutral alumina using hexane:ethylacetate (80:20) as eluent.
The method selectively and efficiently proceeds with active arenas. However, the efforts failed to acylate benzene, chlorobenzene and toluene in the identical condition. Further, it was observed that increasing the alky chain on benzene facilitates the reaction (entry – 9, 10, 11, 12). This finding supports the selectivity of this method for electron donating substrates. We presume that the electrophilic character of acyl chlorides is enhanced by the zinc which in turn reacts at the electron rich position of arenes. This effect may be explained by assuming that the polar groups complex efficiently to the zinc surface9. Though the reaction proceeds 0.5 eq. of zinc, the rate of the reaction is enhanced by employing 1 eq. of zinc. It is important to note that the unused zinc is recovered at the end of the reaction, which can be reused again after activation.
Table:
Arenes Acid chlorides Time/h Yielda
1a 2a 6 90
1a 2b 5 91
1a 2c 7 85
1a 2d 8 89
1b 2a 6 90
1b 2b 6 93
1b 2c 7 85
1b 2a 6 90
1c 2b 7 83
1c 2c 7 80
1c 2d 6 93
1d 2b 7 89
1d 2c 8 92
1d 2d 7 94
[Note from Kinetic: All acyl groups go in the 4-position]
Arenes:
1a. Anisole
1b. N-2,6-dichlorophenyl aniline
1c. Isobutyl benzene
1d. Thiophene
Acyl chlorides:
2a. Pivaloyl chloride
2b. Palmitoyl chloride
2c. p-Chlorobenzoyl chloride
2d. [Rather large acyl chloride with no trivial name]
a: Yields are obtained after column chromatography purification.
References: [Also look quite interesting IMHO]
1. Olah, G.A. ‘Friedel-Crafts Chemistry’, Wiley Interscience, New York and London, 1973
2. Khalikar, B.M.; Borkar, S.D. Tetrahedron Lett. 1997, 38, 1641. b) Izumi, J; Mukaiyama, T. J. Chem. Soc. Chem. Commun, 1996, 183.
3. Izumi, J; Mukaiyama, T. Chem. Lett. 1996, 739 and references cited therein.
4. Clark, J.H.; Keybett , A.P.; MaCquarrie, B.J.; Barlow, S.J.; Landon, P. J. Chem. Soc. Chem. Commun. 1989, 1353.
5. Poul, V.; Sudalai, A.; Daniel, T.; Srinivasan, K.V. Tetrahedron Lett. 1994, 35, 2601. b) Chiche, B.; Finiels, A.; Gauphier, C.; Gauphier, P.; Graille, J.; Piock, D. J. Org. Chem. 1988, 51, 2128. c) Sreekumar, R.; Raghavakaimal Padmakumar. Synth. Comm. 1997, 27, 777.
6. Furstner, A.; Synthesis, 1989, 571 and references cited therein. b)Angew Chem. Int. 1997, 36(3), 186 (Copper), 35(2) 2333 (Titanium).
7. Yadav, J.S.; Srinivas, D.; Reddy, G.S. [A busy bee, one of the authors in Post 407575 (Chimimanie: "Well chief if you ask me", Novel Discourse)]; Himabindu, K. Tetrahedron Lett. (accepted).
8. Meshram, H.M. Tetrahedron Lett. 1993, 34, 2521. b) Meshram, H.M.; Srinivas, D.; Yadav, J.S. Tetrahedron Lett. (accepted).
9. Coates, G.E.; Ridley, D. J. Chem. Soc., 1965, 1870. b) Rao, S.A.; Chao, T.; Schipor, I.; Knochel. P. Tetrahedron, 1992, 48, 2025.
(Hive Bee)
02-17-03 17:46
No 408994
At last... Glad someone liked my post!
Anyway, would activating zinc for this synthesis be as simple as Barium's method of 'stirring in 20ml 5% aq. HCl for two minutes then washed with 3x50ml water and finally 20ml MeOH', scaled to size of course? And would a finer grade of zinc powder be beneficial, or would any decent purity zinc powder suffice?
Also, although it's related to Grignard-type organozinc reactions, a quote from ../rhodium /grignar
the undesireableity of using ether as a solvent in acid chloride reactions (because of ether cleavage and ester formation)
Is there anything to worry about here? Although anisole is acylated without ether cleavage, the methylene ether of benzodioxole is very sensitive to demethyleneation![]() . Anisole can be acylated with AlCl3 as catalyst, whereas benzodioxole can't.
. Anisole can be acylated with AlCl3 as catalyst, whereas benzodioxole can't.
(Hive Addict)
02-17-03 18:36
No 409007
Since I´m currently looking into various Friedel-Craft acylations this was a great reference. Thank you Kinetic!
Yes, I think the simple activation I described in the post you mentioned will suffice. Just make sure to dry the activated zink by washing it with enough dry solvent. Hopefully the method you described will work with anhydrides too. We´ll see soon enough.
Freaky
(Hive Addict)
02-17-03 18:38
No 409008
Don't forget to post your results!
Accept No Imitations, There Can Only Bee One; www.the-hive.ws
(I'm Yust a Typo)
02-17-03 18:51
No 409012
>And would a finer grade of zinc powder be beneficial, or would any decent purity zinc powder suffice?
Reactions like these often depend on the amount of metal surface available. That means that you don't want big lumps of Zn in your reaction mixture. OTOH, if you have _very_ finely powdered zinc powder, it will be oxidized to (inactive) Zn2+ in no-time by HCl. So there's something to balance. 60 to 100 mesh powder often works OK.
(Hive Addict)
02-17-03 18:52
No 409014
-
Accept No Imitations, There Can Only Bee One; www.the-hive.ws
(Hive Bee)
06-25-04 16:02
No 515345
Reactions like these often depend on the amount of metal surface available. That means that you don't want big lumps of Zn in your reaction mixture. OTOH, if you have _very_ finely powdered zinc powder, it will be oxidized to (inactive) Zn2+ in no-time by HCl. So there's something to balance. 60 to 100 mesh powder often works OK.
The first experiments leading to the discovery of Friedel-Crafts reactions as we know it were done using metals, not metal chlorides, as catalysts, and it is assumed that the actual catalytic species were metal chlorides formed in situ. It might well be so that the same applies here and that the formation of ZnCl2 helps - after all, it is a known Friedel-Crafts catalyst.
fear fear hate hate
(Hive Bee)
07-12-04 17:26
No 518919
(Rated as: good read)
Zinc Mediated Friedel–Crafts Acylation in Solvent-Free Conditions under Microwave Irradiation
Satya Paul, Puja Nanda, Rajive Gupta, André Loupyb
Synthesis 2003(18), 2877–2881

Abstract
Zn powder is found to catalyze the Friedel–Crafts acylation of aromatic compounds with acyl halides efficiently under microwave irradiation in solvent-free conditions. Activated substrates undergo acylation predominantly at the para-position. The Zn powder can be re-used up to six times after simple washing with diethyl ether and dilute HCl.
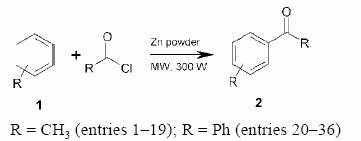
Preparation of Acetophenone, Entry 1; Typical Procedure
To a mixture of benzene (0.078 g, 1 mmol) and Zn powder (0.065 g, 1 mmol) in a 50 mL borosil beaker, AcCl (0.078 g, 1 mmol) was added. The reaction mixture was mixed properly with the help of a glass rod (10 s) and then irradiated in a microwave oven for 30 s at 300 W (monitored by TLC). The reaction mixture was cooled and extracted with Et2O (3 × 10 mL). After drying the ether extracts over anhydrous Na2SO4, the acetophenone was obtained by removal of the solvent under reduced pressure. Finally, the product was purified by column chromatography (silica gel) using P.E. as eluent (0.114 g, 95%).
The reaction was also performed with benzene (0.390 g, 5 mmol), Zn powder (0.325 g, 5 mmol) and AcCl (0.390 g, 5 mmol) in a 50 mL borosil beaker following the same procedure. After an irradiation time of 30 s, acetophenone (0.56 g, 94%) was obtained by passing through column of silica gel and elution with P.E.
The structure of the products was confirmed by 1H NMR, IR and comparison with authentic samples obtained commercially or prepared by reported methods.