08-12-04 03:29
No 525033
Have these two compounds ever been synthesized? Are they psychoactive?
The first is simply Ritalin without the
R-C02Me group while the second is the same compound plus a 3,4-methylenedioxy system attached to the phenyl ring.
How would you make them? Any ideas? Perhaps benzaldehyde (or piperonal) plus 2-MgBr-piperidine followed by removal of the resulting benzyl OH?
(Hive Bee)
08-12-04 07:55
No 525064
I have thought about that compound sometime also, it does have the phenylisopropylamine structure so common among stimulants.
You can only run the grignard with tertiary amines, so your scheme won't work.
Perhaps you could run a Friedel-Crafts alkylation with benzyl chloride and pyridine, and then reduce the resulting compound to the piperidine. Or instead you could react 2-BrMg-pyridine with benzyl chloride, and then reduce.
(Hive Bee)
08-12-04 08:26
No 525066
Unfortunately, I don't have the resources/connections to pull that synthesis off myself. I emailed "Ask DrShulgin" about it hoping that he might be impelled to do it himself, but I seriously doubt he will.
Maybe one of you could pull it off? Bandil or Barium perhaps??? You could conceivably create the next wonder drug (or two)!
(Chief Bee)
08-12-04 14:53
No 525105
A related substance (2-benzhydryl-1-methyl-piperidine) is discussed here: Post 122779 (dormouse: "alpha-cyclohexyl amphetamine analogue -Lilienthal", Serious Chemistry)
Molecule: N-methyl-alpha-benzhydryl-piperidine ("CN3C(CCCC3)CC(C2=CC=CC=C2)C1=CC=CC=C1"
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
08-13-04 04:56
No 525245
(Rated as: excellent)
First, just for the sake of an illustration:
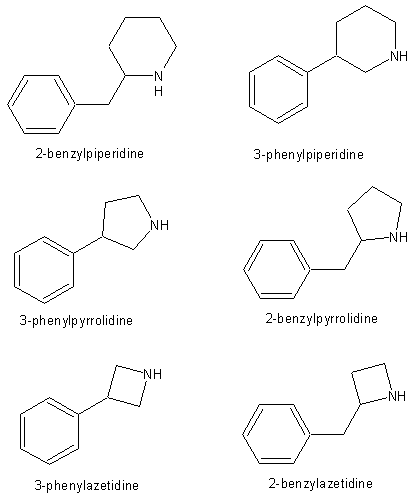
2-benzylpiperidine and 3-phenylpiperidine might have some potentials as stimulants but I seriously doubt about their potential as serotonegics – in my opinion, adding a methylendioxy group to these would not result in MDMA-like activity.
I would also be very skeptical about the alleged amphetamine like activity of alpha-cyclohexyl-PEA mentioned in the link Rhodium provided. Its structure is somewhat similar to lefetamine and it might act trough NMDA blocking and opiate receptors (Post 499450 (Fastandbulbous: "Possible new dissociative (NMDA antagonist)", General Discourse)). Nevertheless it might be an interesting kind of psychoactivity.
Less bulky “ethylamine” side chain would be preferred for serotonergic activity. Reducing the size of the ring to 3-phenylpyrrolidines has more potential for some 5-HT2A affinity, though unfortunately it has even more potential for 5-HT1A affinity. Here is a paper where 3-phenylpyrrolidine based 5-HT1A antagonists show also some nanomolar affinity for 5-HT2A receptors. I assume if one would prepare some suitably substituted 3-phenylpyrrolidines without those annoying N-alkylamide side chains, so typical for 5-HT1A and 5-HT2A antagonists, the resulting compound would be an agonist:
N-Substituted-3-arylpyrrolidines: Potent and Selective Ligands at Serotonin 1A Receptor
Kyo Han Ahn, Seok Jong Lee, Chang-Ho Lee, Chang Y. Hong and Tae Kyo Park
Bioorganic & Medicinal Chemistry Letters 9 (1999) 1379-1384.

Abstract: 3-Arylpyrrolidines are synthesized through the coupling of N-benzyl-3-(methanesulfonyloxy)pyrrolidi
There are also some other papers on 3-phenylpyrrolidine derived compounds and their pharmacology referenced in the paper above (but at the moment I don’t have access to the full texts). Unfortunately none seam to target for 5-HT2A agonist activity:
Regioselective synthesis of 3-aryl substituted pyrrolidines via palladium catalyzed arylation: pharmacological evaluation for central dopaminergic and serotonergic activity.
Sonesson C, Wikstr6m H, Smith MW. Svensson K, Carlsson A, Waters N.
Bioorg. Med. Chem. Lett. 1997:7;241-246. DOI:10.1016/S0960-894X(96)00618-X
Abstract: A series of 3-arylpyrrolidines has been synthesised via palladium catalyzed arylation and evaluated for dopaminergic and serotonergic activity in vitro and in vivo. Compounds substituted by electron withdrawing groups on the meta position of the aromatic ring, were found to be preferential dopamine autoreceptor antagonists.
Basha FZ, DeBernardis JF.
Pure & Appl. Chem. 1994:66;2201-2204.
Hancock AA, Buckner SA, Giardina WJ, Brune ME, Lee JY, Morse PA, Oheim KW, Stanisic DS, Warner RB, Kerkman D J, DeBernardis JF. J.
Pharmacol. Exp. Ther. 1995:272;1160-1169.
Patent DE19615232
Some 3-phenylpyrrolidines seam to have some potential for cocaine-like activity:
Actions of A-75200, a novel catecholamine uptake inhibitor, on norepinephrine uptake and release from bovine adrenal chromaffin cells.
Firestone JA, Gerhardt GA, DeBernardis JF, McKelvy JF, Browning MD.
J Pharmacol Exp Ther. 1993 Mar;264(3):1206-10. Medline (PMID=8450458)
Abstract: The balance between catecholamine (CA) release and reuptake is closely regulated and determines the effective level of transmitter at the synaptic cleft. Drugs that block CA uptake have potential utility as antidepressant medications. One such drug is racemic (+/-)-(1' R*,3R*)-3-phenyl-1-[1',2',3',4'-tetrahyd
Synthesis and pharmacological characterization of ABT-200: a putative novel antidepressant combining potent alpha-2 antagonism with moderate NE uptake inhibition
Zelle RE, Hancock AA, Buchner SA, Basha FZ, Tietje K, DeBernardis JF, Meyer MD.
Bioorg. Med. Chem. Lett. 1994:4;1319-1322. DOI:10.1016/S0960-894X(01)80353-X
Abstract: ABT-200, (±)-(1'R*,3R*)-3-phenyl-1-[(1',2',3',4'-
The stimulant (R)-(+)-alpha,alpha-Diphenyl-2-pyrrolidi
Reducing the ring further down to the azetidine size is in my opinion most promising for hallucinogenic activity. I already posted about that in Post 514788 (Nicodem: "Pharmacology of the 3-phenylazetidines", General Discourse), but that did not raise any interest.
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Hive Bee)
08-13-04 15:01
No 525316
Perhaps condensing benzene (or methylenedioxybenzene) with hypophosphorous acid and 2-carboxylic acid piperidine and then reducing the ketone would work.
BTW what is the IUPAC name for 2-carboxylic acid piperidine? Is it commercially available? If not, how would one make it?
I still have high hopes for alpha-piperonylpiperidine partly because 3,4-methylenedioxy-2-aminoindan doesn't have the traditional amphetamine structure framework but is still serotonergic, which is not to say that your idea about the reduced ring size analogues should not be investigated as well because I would like to do that as well.
Any takers?
(Hive Bee)
08-14-04 02:14
No 525381
(Rated as: good read)
Perhaps condensing benzene (or piperonal) with hypophosphorous acid and 2-carboxylic acid piperidine and then reducing the ketone would work.
I doubt you can acylate benzene or benzodioxole with piperidine-2-carboxylic acid (that’s its IPAC name
I still have high hopes for alpha-piperonylpiperidine partly because 3,4-methylenedioxy-2-aminoindan doesn't have the traditional amphetamine structure framework but is still serotonergic
Well, the aminoindane analogue is actually the less bulky one. The indane ring is fixed almost in one plane and its conformers don’t expand far from the benzene ring. As you can se bellow the conformers of the MDMA molecule can wag on a notably larger area (blue circle) and also considerably out of the benzene plane (not shown). Obviously the space required for the 2-benzylpiperidine to wag from different conformers to the potentially active conformation is quite larger. The restricted receptor site size might not allow for such changes.
Given that the aminoindane analogue is active* we can assume that the preferred ethylamine side chain conformation is to be in plane with the benzene ring (psychedelic amphetamines – 5-HT2A agonists – have very different requirements though). Also, the alpha methyl group in MDMA does not pose much steric interference to the acceptor group in the receptor site (red arc), while in the 2-benzylpiperidine the piperidine ring makes quite an interference and limits the access from many directions. The steric repulsion with the benzene ring would also make any conformation of the ethylamine chain, similar to the required one, energeticaly unfavorable.
You must also consider that the receptor site pockets often don’t allow much space and sometimes already an addition of a methyl group causes the loss of activity. As you probably know MBDB, the aminobutane analogue of MDMA, is slightly weaker and lacks the stimulant activity* and as far as I know the aminopentane analogue is probably already inactive. Now, the 2-benzylpiperidine analogue would have similar space requirements like the aminopentane analogue if not higher. Keep also in mind that the phentermine MDMA analogue (alpha-methyl-MDMA) is already inactive* even though it differ from MDMA by only one methyl group.
Furthermore the inertia of the piperidine ring is higher and the interaction with the acceptor molecule in the receptor site is more likely to lose grip much easier than with MDMA and obviously much, much easier than with the conformationaly restricted aminoindane.
I’m not saying that it would be useless to prepare and test the compound in question. It is quite possible that it is psychoactive. There are many other sites with which it might interact, like the dopamine receptors probably. I’m just saying that its interaction with the serotonin transporter in a MDMA-like fashion as well as with other relevant sites is not very feasible.
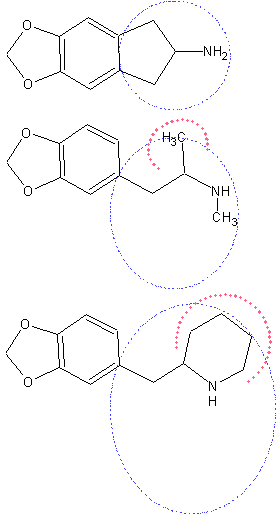
Further read:
* Structure-Activity Relationships of MDMA-Like Substances.
Nichols, Oberlender
http://www.nida.nih.gov/pdf/monographs/d
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Hive Bee)
08-14-04 02:34
No 525384
IUPAC Name: Pipecolic acid
CAS Number: 535-75-1 (the (R)-form)
CAS Name: 2-Piperidinecarboxylic acid
IUPAC Name: Piperidine-2-carboxylic acid
Additional Names: Pipecolinic acid, Hexahydropicolinic acid, Homoproline, Dihydrobaikianine and many others
Price:
5 gr ~ $15-20
25 gr ~ $40-70
The l-form occurs in plants: Phillips, Chem. & Ind. (London) 1953, 127.
Synthesis:
A. Ladenburg, Ber. 24, 640 (1891)
Stevens, Ellman, J. Biol. Chem. 182, 759 (1950)
V. Asher et al., Tetrahedron Letters 22, 141 (1981)
Synthesis of L-pipecolic acid from L-lysine: Fujii, Miyoshi, Bull. Chem. Soc. Japan 48, 1341 (1975) - actually biosynthesis
Synthesis of racemate: R. T. Shuman et al., J. Org. Chem. 55, 738 (1990)
(Hive Bee)
08-14-04 20:58
No 525471
Thanks for all the input.
So, if one were to try to see if the condensation reaction will be a go even without N protection, what procedure to use?
I have extrapolated this rough first draft based on the DOAM entry in PiHKAL. I'm pretty sure that I got the stoichiometries right. Note: this is a highly experimental procedure write up and has not been attempted.
SYNTHESIS: A solution of 62.4 g benzene (or 97.6 g
1,3-benzodioxole) and 129 g pipecolic acid in 168 g polyphosphoric acid are to be heated on a steam bath for 3 hours. The strongly acidic solution is then to be poured into 1 L of water and extracted (how to extract without basifying enough to cause intramolecular imine formation while still getting rid of the water soluble acids is eluding me--Shulgin wasn't dealing with an amine and an amine with a ketone functionality at that in his procedure).
After that little kink is worked out, the pure ketone is to presumably undergo the following. To 360 g mossy zinc there is to be added a solution of 7.2 g mercuric chloride in 200 mL warm water, and this solution is to be swirled periodically for 2 hours. The water is to be drained off, and the amalgamated zinc added to a 2 L three-necked round bottomed flask, treated with 200 mL concentrated hydrochloric acid, and heated with an electic heating mantle. Next, a solution of either 48.4 g of the benzene based ketone or 51.5 g of the piperonyl based ketone in 107 mL ethyl alcohol containing 30 mL concentrated hydrochloric acid is to be added dropwise over the course of 4 hours accompanied by 350 mL concentrated hydrochloric acid added batchwise over the same period. The mixture is to be refluxed overnight, cooled and diluted with enough water to make methylene chloride be the lower phase (on bottom). The phases are to be separated using a seperatory funnel and the aqueous phase extracted with two 200 mL increments more of methylene chloride. Next, the two organic phases are to be combined, washed first with 5% sodium hydroxide, and then with water, and the solvent removed under vacuum. The mother liquors are then distilled and the higher boiling of the 2 (presumably) fractions that come over saved, gassed with HCl or what not, and spread out under the heat lamp to dry.
Help! This project needs more practical advice. Thanks.
Oh yeah, can a methylenedioxybenzene ring system stand all the concentrated hydrochloric acid required in Step 2 (the Clemmenson reduction)? If it can then that means they can make mdma from methylone, I suppose.
It would probably also be better to use racemic pipecolic acid if that is available. I don't know if the R form is going to give the right or the wrong or a mixture of both products. Does anyone know the stereochemical configuration of Focalin (dexmethylphenidate)?
(Hive Bee)
08-15-04 03:24
No 525507
Cattleprodder, maybe you are not aware that polyphosphoric acid is one of the most powerful dehydrating reagents. This means that there is no way pipecolic acid would acylate benzene without side reactions. If you don’t protect that secondary amine, either as a triflate or as tertiary amine, you would more likely end up with an ugly tar-like polymerization product that could barely deserve to be called a coagulated polypeptide
Secondary aminoketones are usually enough stable as freebases to allow for the extraction.
Anyway, let’s suppose you try with N-methyl-pipecolic acid and somehow end up with the N-methyl-2-benzoylpiperidine. Then you have the problem of the Clemmensen not working well with aminoketones. I have yet to see a successful Clemmensen for alpha-aminoketones. Even aminoketones with the amine group on a more distant position still give rearrangement products in some cases. A more promising method might be the Wolf-Kishner reduction.
However, don’t you think that before jumping on the synthesis propositions it might bee cleverer to first do a literature search for the pharmacology of the compound and its closest derivatives? I gave you a hint that these compounds are known and there is literature available for both synthesis and pharmacology. I was expecting you would follow that hint and post some really interesting info, since obviously they interest me as well (but I should be studying for an important exam instead of searching for literature
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Hive Bee)
08-22-04 13:46
No 526803
Rhodium posted about pyrrolidinopropiophenones which have supposedly appeared on the illicit drug market, including 4'-Methoxy-pyrrolidinopropiophenone (MOPPP) and 3',4'-Methylenedioxy-pyrrolidinopropioph
There are some additional papers along the same lines for additional analogs alpha-pyrrolidinopropiophenone (PPP), 4'-methyl-alpha-pyrrolidinopropiophenone
Molecule: 4'-Methoxy--pyrrolidinopropiophenone (MOPPP) ("O=C(C1=CC=C(OC)C=C1)C(N2CCCC2)C")
Molecule: 3',4'-Methylenedioxy-pyrrolidinopropioph
Molecule: 4'-Methyl-alpha-pyrrolidinopropiophenone
Molecule: 4'-Methyl-alpha-pyrrolidinohexanophenone
With the oxygen and nitrogen maybe this look a little bit alike
Molecule: alpha-pyrrolidinopropiophenone (PPP) ("O=C(C1=CC=CC=C1)C(N2CCCC2)C")
Molecule: 4-methylaminorex ("CC1N=C(N)OC1C2=CC=CC=C2")
______ _____ ____ ___ __ _
Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: a synopsis.
Dietmar Springer, Giselher Fritschi, Hans H. Maurer, R. Staack.
Ther. Drug Monitor. 26(2), 127-31. (2004)

Abstract
Designer drugs of the amphetamine type (eg, MDMA, MDEA, MDA), of the new benzyl or phenyl piperazine type (eg, BZP, MDBP, mCPP, TFMPP, MeOPP), or of the pyrrolidinophenone type (eg, PPP, MOPPP, MDPPP, MPPP, MPHP) have gained popularity and notoriety as rave drugs. These drugs produce feelings of euphoria and energy and a desire to socialize. Although in the corresponding drug scene designer drugs have the reputation of being safe, studies in rats and primates in combination with human epidemiologic investigations indicate potential risks to humans. Thus, a variety of adverse effects have been associated with the use/abuse of this class of drugs in humans, including a life-threatening serotonin syndrome, hepatotoxicity, neurotoxicity, and psychopathology. Metabolites were suspected to contribute to some of the toxic effects. Therefore, knowledge of the metabolism is a prerequisite for toxicologic risk assessment. The metabolic pathways, the involvement of cytochrome P450 isoenzymes in the main pathways, and their roles in hepatic clearance are described for designer drugs of different groups. In summary, polymorphically expressed CYP2D6 was the major enzyme catalyzing the major metabolic steps of the studied piperazine- and pyrrolidinophenone-derived designer drugs. However, it cannot be concluded at the moment whether this genetic polymorphism is of clinical relevance.
______ _____ ____ ___ __ _
Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4'-methyl-alpha-pyrrolidinopropiophenone
Dietmar Springer, Giselher Fritschi and Hans H. Maurer
Journal of Chromatography B 796(2), 253-66 (2003)
Medline (PMID=14581066)
Abstract
R,S-alpha-pyrrolidinopropiophenone (PPP) is a new designer drug with assumed amphetamine-like effects which has appeared on the illicit drug market. The aim of this study was to identify the PPP metabolites using solid-phase extraction, ethylation or acetylation as well as to develop a toxicological detection procedure in urine using solid-phase extraction, trimethylsilylation and gas chromatography-mass spectrometry (GC-MS). Analysis of urine samples of rats treated with PPP revealed that PPP was extensively metabolized by hydroxylation of the pyrrolidine ring with subsequent dehydrogenation to the corresponding lactam, hydroxylation of the aromatic ring in position 4' or double dealkylation of the pyrrolidine ring to the corresponding primary amine (cathinone) partly followed by reduction of the keto group to the corresponding secondary alcohol (norephedrines). As cathinone and the norephedrine diastereomers are also formed after intake of other drugs of abuse or medicaments, special attention must be paid to the detection of the unequivocal metabolite 2"-oxo-PPP as an unambiguous proof for the intake of PPP. The hydroxy groups were found to be partly conjugated. Based on these data, PPP could be detected in urine via its metabolites by full-scan GC-MS using mass chromatography for screening and library search for identification by comparison of the spectra with reference spectra. The same toxicological detection procedure can be applied to other designer drugs of the pyrrolidinophenone type, like MOPPP, MDPPP, MPHP, and MPPP. The detection of the latter will also be presented here.
______ _____ ____ ___ __ _
Studies on the metabolism and toxicological detection of the new designer drug 4'-methyl-alpha-pyrrolidinopropiophenone
Dietmar Springer, F. Peters, Giselher Fritschi and Hans H. Maurer
Journal of Chromatography B 773(1), 25-33 (2002)
Medline (PMID=12015267)
Abstract
4'-Methyl-alpha-pyrrolidinopropiophenone
______ _____ ____ ___ __ _
New designer drug 4'-methyl-alpha-pyrrolidinohexanophenone
Dietmar Springer, F. Peters, Giselher Fritschi and Hans H. Maurer
Journal of Chromatography B 789(1), 79-91 (2003)
Medline (PMID=12726846)
Abstract
R,S-4'-Methyl-alpha-pyrrolidinohexanophe
boot from the shadow of a broken mirror
(Hive Bee)
08-25-04 17:58
No 527379
Nicodem, for the modification of the heterocyclic ring of 2-benzylpiperidine, I'm not sure quite how effective the structures without the phenylisopropyl structures would be (eg 3-phenylpiperidine). After searching several papers, I found a similar compound, which was stated to be a dopamine reuptake inhibitor (2-methyl-3-phenylquinuclidine), but as you can see, it has the required phenylisopropylskeleton.
Anyhow, main point:
From a paper about the effectiveness of analogues of pipradrol (will post ref when I find the CD-R I've stored it on)with regard to replacing one of the phenyl groups with other aryl compounds (2-thienyl etc), the hydroxy group with a hydrogen and using different nitrogen heterocyclics (ie replacing the piperidyl group with other heterocyclics).
They eventually discovered that the structure given below was the most potent from a wide range of substitutions
The advantage of the morpholines is that they can be made from aminoalcohols and 2-iodoethanol. Using that as a starting place, the appropriate aminoalcohol can be made from reducung diphenylalanine to diphenylalaninol. For the compound analogous to benzylpiperidine, it can be made by reducing phenylalanine to phenylalaninol (3-phenyl-2-aminopropanol), reacting it with 2-iodoethanol to give 3-phenyl-2-(2-hydroxyethyl)propanol, then finally dehydrating with conc sulphuric acid to give 3-benzylmorpholine
(the last two steps are from synthesis of phenmetrazine from phenylpropanolamine).
Although diphenylalanine might not be the easiest amino acid to obtain (for most potent derivative), phenylalanine is available from so many sources it's not worth listing them, and is cheap enough to allow for the synth of 3-benzylmorpholine for evaluation purposes
That is right, the Mascara Snake: Fast and bulbous
(Newbee)
08-28-04 14:37
No 528000
... it can be made by reducing phenylalanine to phenylalaninol (3-phenyl-2-aminopropanol), reacting it with 2-iodoethanol to give 3-phenyl-2-(2-hydroxyethyl)propanol, then finally dehydrating with conc sulphuric acid to give 3-benzylmorpholine ...
I think you mean "3-phenyl-2-(hydroxyethyl)aminopropanol" ![]()
Diphenylmethane can be synthesized from the Friedel-crafts alkylation of benzene in the prescence of DCM and AlCl3. Morpholine can be synthesized from the simple dehydration of diethanolamine which can be obtained from any photography store. Surely there is some way to attach the amine to that alpha-carbon to obtain the target compound; but I'm too tired to think about it...
When you say this particular compound is the most potent, do you mean in a psychedelic (5-HT2x binding, etc.), or in a stimulatory sense? I assume it is stimulatory due to the striking similiarity to methylphenidate.. blah, I can just go to my shrink for that; I even get the isolated dextro isomer all by it's lonesome.
- phenethylman -
(Hive Bee)
08-31-04 19:25
No 528671
Yes, I meant 3-phenyl-2-(2-hydroxyethyl)aminopropanol
That is right, the Mascara Snake: Fast and bulbous
(Newbee)
08-31-04 21:10
No 528742
wow.. now that's potency in a stimulant.. you are right that doesn't sound worthless; it sounds down-right lethal!
.. and I thought I had a meth problem. There's no doubt I would kill myself w/that. Hence the reason my ventures into clandestine chemistry are strictly limited to the psychedelic genre..
good luck.
- phenethylman -
(Hive Bee)
09-01-04 15:24
No 528954
Searching the Combined Chemical Dictionary for 2-benzylpiperidines with MW < 350 revealed 5 interesting compounds. Two of them are very well known - methylphenidate and pipradrol. The other three:
Phenyl-2-piperidylmethanol - antidepressant, anorexic agent; the O-Ac derivative of the (-)-threo-form is called Levofacetoperane or Phacetoperane, the hydrochloride - Lidepran with LD50(rat, orl) 400 mg/kg and LD50(rat, inv) 80 mg/kg.
Rimiterol (α-(3,4-Dihydroxyphenyl)-2-piperid
Difemetorex (2-(Diphenylmethyl)-1-piperidineethanol)
That`s all for now. Tomorrow there will be results for 3-phenylpiperidine - and I`m sure they`ll be rather interesting
(Chief Bee)
09-03-04 09:14
No 529371
(Rated as: excellent)
Zentralerregende Wirkung eines neuen Piperidinderivates
J. Tripod, E. Sury, K. Hoffmann
Experentia 10, 261-262 (1954) (../rhodium/pdf /diphenylmeth
Abstract
A new piperidine derivative, the 2-diphenylmethyl piperidine already in doses of 0.001 g/kg s.c. strong increase of the spontaneous motility of various animals. On mice it has about the same potency as desoxy-ephedrine and is about 3 times more potent than amphetamine. It also acts markedly antagonistic on various narcotics but has practically no peripheral sympathomimetic effects.
____ ___ __ _
Über Alkylenimin-Derivate. II. Mitteilung.
Piperidin-Derivate mit zentralerregender Wirkung. I.
E. Sury und K. Hoffmann
Helv. Chim. Acta. 37, 2133-2145 (1954) (../rhodium/pdf /diphenylmeth
Abstract
A series of new piperidine derivatives substituted in the 2-position has been prepared and tested pharmacologically. Most of these compounds have central stimulating effects, above all the 2-diphenylmethyl-piperidine hydrochloride.
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
09-08-04 11:40
No 530333
(Rated as: good read)
Central Stimulants. α,α-Disubstituted 2-Piperidinemethanols and 1,1-Disubstituted Heptahydrooxazolo[3,4-a]pyridines
Frederick J. McCarty, Charles H. Tilford, M. G. Van Campen
J. Am. Chem. Soc. 79, 472-480 (1957) (../rhodium/pdf /diphenyl-2-p
Abstract
A series of α,α-disubstituted-2-pyridineme
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
09-09-04 07:00
No 530494
(Rated as: excellent)
Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4'-methyl-alpha-pyrrolidinopropiophenone
Dietmar Springer, Giselher Fritschi and Hans H. Maurer
J. Chrom. (B), 2003, 796(2), 253-266
Medline (PMID=14581066)

Abstract: R,S-alpha-pyrrolidinopropiophenone (PPP) is a new designer drug with assumed amphetamine-like effects which has appeared on the illicit drug market. The aim of this study was to identify the PPP metabolites using solid-phase extraction, ethylation or acetylation as well as to develop a toxicological detection procedure in urine using solid-phase extraction, trimethylsilylation and gas chromatography-mass spectrometry (GC-MS). Analysis of urine samples of rats treated with PPP revealed that PPP was extensively metabolized by hydroxylation of the pyrrolidine ring with subsequent dehydrogenation to the corresponding lactam, hydroxylation of the aromatic ring in position 4' or double dealkylation of the pyrrolidine ring to the corresponding primary amine (cathinone) partly followed by reduction of the keto group to the corresponding secondary alcohol (norephedrines). As cathinone and the norephedrine diastereomers are also formed after intake of other drugs of abuse or medicaments, special attention must be paid to the detection of the unequivocal metabolite 2"-oxo-PPP as an unambiguous proof for the intake of PPP. The hydroxy groups were found to be partly conjugated. Based on these data, PPP could be detected in urine via its metabolites by full-scan GC-MS using mass chromatography for screening and library search for identification by comparison of the spectra with reference spectra. The same toxicological detection procedure can be applied to other designer drugs of the pyrrolidinophenone type, like MOPPP, MDPPP, MPHP, and MPPP. The detection of the latter will also be presented here.
______ _____ ____ ___ __ _
Studies on the metabolism and toxicological detection of the new designer drug 4'-methyl-alpha-pyrrolidinopropiophenone
Dietmar Springer, F. Peters, Giselher Fritschi and Hans H. Maurer
J. Chrom. (B), 2002, 773(1), 25-33
Medline (PMID=12015267)

Abstract: 4'-Methyl-alpha-pyrrolidinopropiophenone
______ _____ ____ ___ __ _
New designer drug 4'-methyl-alpha-pyrrolidinohexanophenone
Dietmar Springer, F. Peters, Giselher Fritschi and Hans H. Maurer
J. Chrom. (B), 2003, 789(1), 79-91
Medline (PMID=12726846)

Abstract: R,S-4'-Methyl-alpha-pyrrolidinohexanophe
The tendency is to push it as far as you can
(Hive Bee)
09-10-04 04:31
No 530624
(Rated as: excellent)
Please excuse, A lot of my post will be repeat material / (plagiarised from other posts). Also not all of it has to do with the main topic of the thread. However in this post there are structures of heterocyclic stimulants that relate to this thread a greater or lesser degree (e.g. Phacetoperane). It might be useful to have the diagrams of various non-conventional stimulants in one place (along with a brief description), and be able to spin them skeletons around with the mouse (unless the structures crash the molecule viewer and I have to post a flat picture instead!). Also I think there are a couple structures here that weren't yet mentioned too much elsewhere.
Molecule: diphenyl(piperidin-2-yl)methanol ("OC(C2=CC=CC=C2)(C3=CC=CC=C3)C1NCCCC1")
aka Pipradol, aka Meratran. The "parent" compound. This is legally restricted in sports competition and some countries for modest abuse potential. Sold in tonic.
"Marketed in 1956. Considered to be a mild central nervous system stimulant. Occasionally overstimulation reported. Has been studied in depressive mood. Dosage 1 - 3mg/day."
http://home.intekom.com/pharm/adcock/ale
http://www.psychotropics.dk/alphaindex/v
The compound without the alcohol group, 2-diphenylmethyl piperidine, has also been mentioned in the paper: "in doses of 1 mg/kg s.c. produces a strong increase of the spontaneous motility of animals. On mice it has about the same potency as methamphetamine and is about 3 times more potent than amphetamine but has practically no peripheral sympathomimetic effects". Also pointed out in 'Future Drugs of Abuse': "A best bet for a future CsA is the most potent adrenomimetic compound in this series, 2-diphenylmethylpiperidine."
Molecule: (R)-(+)-alpha,alpha-Diphenyl-2-pyrrolidi
This is obviously a close analog of pipradol. One Hive post said "5-10 mg lasts hours, but not fun", another said "20 mg of the (R) isomer (orally) provides quite a mild, but prolonged stimulation (12 hours) which is quite similar to about 30-40mg of methylphenidate... 20mg IM is 1.5 to 2x oral potency, but it's a lot more euphoric. It also has very little peripheral actions (no heart pounding out of your chest & very little dry mouth)." Apparently works by Dopamine Reuptake Inhibition, like cocaine.
DAT uptake inhibition: Ki is 170 nM vs Cocaine 200 nM
Molecule: (4-(1,2-diphenylethyl)pyridine ("C1(CC(C3=CC=CC=C3)C2=CC=NC=C2)=CC=CC=C
Another similar structure; This was from the thread containing Post 475625 (silenziox: "18 & 23 Cas numbers???", General Discourse)
Another Monoamine Reuptake Inhibitor; it has test tube potency comparable to cocaine (but not aware of any bioassays):
DAT Uptake Inhibition: Ki is 263 nM; 5HT Uptake Inhibition: Ki is 910 nM; NE Uptake Inhibition: Ki is 393 nM
Molecule: 2-benzhydryl-3-methoxy-1-methylpiperidin
Substituted 2-diphenylmethylpiperidine has similar potency: "Minimal cardiac and respiratory effects at stimulant doses and was tried clinically. It was a potent stimulant, but the dose-response effect was too marked. At a given dose, due to the variation in the individual patient response, the level of stimulation attained frequently was above or below that desired." Stimulant at 1 mg/kg ip. N-desmethyl analog: Stimulant at 0.3 mg/kg"
Molecule: alpha,alpha-diphenyl-3-morpholinemethane
Fastandbulbous states it's a stimulant (DAT inhibitor, as is methylphenidate and cocaine), but it was found to be active at a much lower dose than the other two, (reckoned to be 0.5 - 1mg in man). Lillienthal listed the reference for the synthesis on his references page:
Central Stimulants. Cyclized Diphenylisopropylamines
Stanley O. Winthrop and Leslie G. Humber,
J. Org. Chem. 26(8), 2834-2836 (1961)

Molecule: 3-methyl-2-phenylmorpholine ("CC(NCCO2)C2C1=CC=CC=C1")
Phenmetrazine, aka Preludin. A post on the Hive says "Apparently you have to inject phenmetrazine to feel it's true euphoria... When you do that it apparently kicks amphetamine's ass. It was abused much in Sweden in the 60-70s". Oral dosage 50mg 2-3 times a day. Another post states "stimulate the release of Norepinephine like U4Euh does; notice the structural similarity also". Paper says "One widely used approach for the synthesis of new compounds that possess some type of central nervous system stimulant activity is the cyclization of substituted phenethanolamines into heterocycles, such as morpholine, piperidine, and 2-oxazoline in such a manner that more or less of the phenethanolamine skeleton becomes part of the heterocyclic ring. Well-known drugs of this type are phenmetrazine. pipradol, and anminorex."
Post 429511 (alphacentauri: "Phenmetrazine", Stimulants)
Molecule: Phenyl-2-piperidylmethanol ("OC(C2=CC=CC=C2)C1NCCCC1")
aka Phacetoperane: antidepressant, anorexic agent. "Shulgin (1975) suggested that levophacetoperane could well be a future clandestine CsA. However, this compound shares the same limitation as methylphenidate, in that only one diastereoisomer is active; and it is less potent than methylphenidate" Again has the phenethanolamine in heterocycle.
Molecule: 1-(1-benzylbutyl)pyrrolidine ("CCCC(N2CCCC2)CC1=CC=CC=C1")
"Prolintane hydrochloride is a mild central stimulant and has properties similar to those of dexamphetamine. Peak plasma level 1 to 2 hours after single dose. Elimination half-life about 2 hours." There is also the closely related Pyrovalerone:
Molecule: 4'-methyl-2-(1-pyrrolidinyl)valerophenon
"Pyrovalerone, Studied as psychostimulating agent in the management of asthenic and depressive states with mental exhaustion." These two above (prolintane and pyrovalerone) could be called the "parent" molecules for the pyrrolidinopropiophenones posted above, synthesized by Nemo Tenetur.
Molecule: 4-(4-chlorophenyl)-1-methyl-3-propylpipe
This molecule could be considered a piperidine based cocaine analog (or more closely, phenyltropane analog) It is mentioned in ../rhodium/pdf /cocaineanalog
Binding to DAT (cis isomer): Ki 3.0 nM; DAT Inhibition (cis isomer): Ki 8.3 nM
Molecule: (S)-methyl 2-(3,4-dichlorophenyl)-2-((S)-piperidin-
Here is a halogenated Methylphenidate analog with greater potency than the parent compound (mentioned in the same review paper as the piperidine derivative above.)
DAT Binding: Ki 5.3 nM; DAT Inhibition: Ki 7.0 nM
Molecule: 3-(3,4-dichlorophenyl)-N-methyl-1-indana
aka Indatraline, Lu 19-005
According to preliminary investigations, potential antidepressant agent. Very long-lasting and potent cocaine-like stimulant, self administered in monkeys but with long intervals in between, because of long duration of action. Produces stereotypies, weight loss, anemia similar to those caused by chronic high-dose cocaine. Combines an equally potent inhibitive effect on dopamine, norepinephrine and serotonin.
Molecule: 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydr
aka Diclofensine.
NE/5-HT/DA uptake inhibitor. Psychostimulating and mood elevating agent. Double-blind clinical trials in the treatment of depressions and dysphoric mood. Minor anticholinergic effects. Dosage 25 to 150mg/day. Plasma half-life in healthy subjects about 4 hours. Related to Nomifensine, which itself had recreational and motivating potential and which many people liked before it was removed from the pharmaceutical market.
1-(3,4-dichlorophenyl)-3-azabicyclo-[3.1
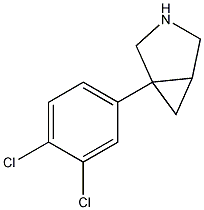
aka DOV 21,947. For some reason this bicyclic structures seems to crash the molecule viewer. Inhibits the reuptake of serotonin, norepinephrine, and dopamine (IC50 values of 12, 23, and 96 nM, respectively). DOV 21,947 reduces the duration of immobility in the forced swim test (using rats) with an oral minimum effective dose of 5 mg/kg. This antidepressant-like effect manifests in the absence of significant increases in motor activity at doses of up to 20 mg/kg. DOV 21,947 also produces a dose-dependent reduction in immobility in the tail suspension test, with a minimum effective oral dose of 5 mg/kg. Although this may not be a locomotor stimulant it looks like an interesting / promising antidepressant, also it has the similar structural features of this thread.
Antidepressant-like actions of DOV 21,947: a "triple" reuptake inhibitor
Phil Skolnicka, Piotr Popikb, Aaron Janowskyc, Bernard Beera, Arnold S. Lippa,
European Journal of Pharmacology. 461, 99–104 (2003)
DOI:10.1016/S0014-2999(03)01310-4

______ _____ ____ ___ __ _
As a side note: there seem many stimulant molecules where the 4-halogen or the 3,4-halogen on the phenyl confers added potency. 4-fluoroamphetamine seems to be an interesting variation on amphetamine with slightly more sertonergic character. I wonder if 3,4-fluoroamphetamine would be even more serotonergically interesting. Which brings to mind (getting totally off track,) I think 3-methoxy-4-ethoxyamphetamine would be like MDA / MDMA. Look at pihkal 123 (MEPEA, 3-methoxy-4-ethoxyphenethylamine, lightly active at 300 mg "The effects were very quiet, very pleasant, and very light. There was nothing psychedelic here, but rather a gentle lifting of spirits") compared to pihkal 115 (MDPEA, 3,4-methylenedioxyphenethylamine, inactive at 300 mg). It stands to reason that the already-active MEPEA would become more potent and empathogenic effects accentuated as it followed the analogous path from MDPEA to MDA to MDMA. Also ME (metaescaline, 3,4-dimethoxy-5-ethoxyphenethylamine) seems to exhibit some "empathogenic" qualities, maybe the 3-ethoxy-4-methoxy-amphetamine would also. Additional interesting to look at would be MEA 3-methoxy-4-ethylamphetamine, similar to 3-methoxy-4-methylamphetamine MMA, and 3-methoxy-4-bromoamphetamine (with the last few, have to be careful of PMA trouble or PCA neurotoxicity of course).
______ _____ ____ ___ __ _
N-ethyl-3-phenylbicyclo[2.2.1]heptan-2-a
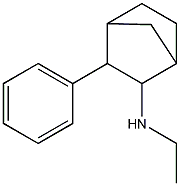
aka Fencamfamine. From the Hive "I have heard from a talented friend of mine who has exceptional taste that fencamfamine is his favorite stimulant of them all..." and another post mentioned "Fencamfamine (FCF) is a CNS stimulant that works by blockading dopamine reuptake and it has a potency similiar to amphetamine. Well that's what i was told by my coach who sold it to me, lol! I took a 0.5g IV dose for a research experiment once, that produced a very clean & subtle buzz which lasted for about 4-5 hours from memory. Nothing special, but I found sex was far more pleasurable using FCF as opposed to rooting on meth." Adding halogen 4 or 3,4 on the phenyl may further increase potency.
methyl 3-(4-bromophenyl)-8-methyl-8-aza-bicyclo
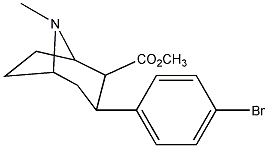
aka RTI-51 (para-iodo is RTI-55, equally potent). These are basically the most potent 3-phenyltropanes, with description of effects very similar to indatraline (very long lasting (16 h), very potent, reinforcing, locomotor stimulant, and substitute for cocaine). "During the first 2 h of behavioral observation that followed the intraperitoneal (IP) administration of the compound, each of the 3-phenyltropane analogs and cocaine increased horizontal activity). Cocaine, at 30.0 and 56.0 mg/kg, produced significantly greater activity than saline, with a peak effect at approximately 36 000 counts; RTI-51, at 0.49 mg/kg, significantly increased activity to 51 000 counts. RTI-51 (0.49 mg/kg) had an AUC significantly greater than that of 3.0 mg/kg cocaine. When the peak of the activity time course of the phenyltropane analogs were compared with that of cocaine, only 0.49 mg/kg RTI-51 produced a significantly greater peak effect than 3.0 mg/kg cocaine did. In rats, RTI-55 increased extracellular dopamine levels in the nucleus accumbens to about 350% of control values."
Molecule: R-1-(Benzofuran-2-yl)-2-propylaminopenta
aka (-)-BPAP. This compound is extremely interesting, has a number of unique activities and all of them positive. Also quite potent. Is not only catecholaminergic and serotonergic activity enhancer, but also a norepinephrine and dopamine uptake inhibitor and a weak serotonin uptake inhibitor, without adverse MAOI hypertensive crisis potential. [3H]dopamine, [3H]norepinephrine, and [3H]serotonin uptake IC50 were 42 nM, 52 nM, and 640 nM respectively; thus the effects of (-)-BPAP on dopamine and norepinephrine uptake were more potent than those of cocaine, while its potency at inhibiting serotonin uptake was weaker than that of cocaine. The uptake inhibitory effect of (-)-BPAP may be involved in motor stimulant effects in addition to its Catecholamine Enhancing and Serotonin Enhancing effect. In normal non-habituated rats, BPAP HCl increased motor activity during the entire 2-h observation period.
Post 211596 (Rhodium: "BPAP, a catecholamine activity enhancer", General Discourse)
Molecule: 7-benzyl-1-ethyl, 4-oxo 1,8-naphthyridine-3-carboxylic acid ("O=C(C1=CN(CC)C2=C(C=CC(CC3=CC=CC=C3)=N
aka amfonelic acid; cocaine-like stimulant with apparently very euphoric or addictive qualities. The rats go absolutely nuts for this one. Interesting that this stimulant looks unlike most other stimulant families, although still has phenalkylamine skeleton. In fact it is more closely related to antibacterial compounds. Should be pretty potent in humans, maybe 10 mg or less.
DA, NE, 5HT Uptake Inhibition: 7.2 nM, 41.1 nM, 879 nM
1-(1-(benzo[b]thiophen-2-yl)cyclohexyl)p
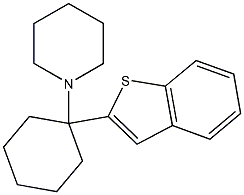
aka BTCP. This is a PCP analog but instead of being a dissociative it is a very specific cocaine-like stimulant compound, like amfonelic acid. The rats love this compound too. However, there are some health problems observed after repeated i.p. injections. In vivo Potency is comparable to cocaine; active metabolites may contribute to in vivo effect. "The reinforcing actions of BTCP were compared to those of cocaine at equimolar concentrations in drug-naive rats. Two doses (0.125 and 0.25 mg/infusion) that produced reliable self-administration in all the animals for cocaine and BTCP were then tested on a progressive-ratio schedule. At each dose, BTCP supported higher breaking points (BPs) than cocaine. The results demonstrate that rats readily acquire responding maintained by BTCP and suggest that BTCP may have greater reinforcing effects than cocaine at equimolar concentrations."
DA-uptake inhibition: IC50 11 nM vs. 296 nM for cocaine
Further Studies of the Structure-Activity Relationships of 1-[1-(2-Benzo[b]thienyl)cyclohexyl]piper
Xiao-shu He, Lionel P. Ramon, Mariena V. Mattson, Mohyee E. Eldefrawi and Brian R. de Costa,
J. Med. Chem. 36(25), 4075-4081 (1993)

Also Post 99112 (beagle boy: "Re: PCP Synthesis", Chemistry Discourse) beagle boy said: "The cpd was synthesized (not by me of course) and bioassayed by vaporizing and inhaling it in very small quantities (again, not by me). No effects were apparent, but in retrospect the amounts assayed were quite small, maybe on the order of 10-20 mgs or so."
Post 435648 (pHarmacist: "BTCP - a compound with cocaine-like properties", Novel Discourse) Two nice refs by pHarmacist
Post 507205 (7is: "More PCP related studies", Methods Discourse) Some more nice refs by 7is
1-(benzo[b]thiophen-2-yl)-N-propylcycloh
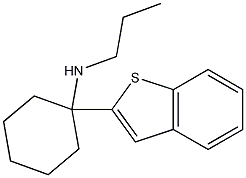
This BTCP analog might be about 2x more potent than BTCP based on binding and DAT inhibition.
DA-uptake inhibition: IC50 5 nM
I have some more relevant references for these notes but I am too tired (read lazy) to retrieve them, check if they've been posted already, upload them, type up the reference in the proper format, and place them next to the appropriate location. This post is, even without them, a spacewasting bewhhymouth.
If references are desired, I'll do what I can, if I can, when I can, and where I can (probably a subsequent post). Which, by the way, I live in a can -- and can openers are the definite alphanumerical warnings. Which is to say, watch out for metallic indentations, the sky might crack open and blaze terrible light at any moment. What they didn't tell you about judgement day is that you get et.
boot from the shadow of a broken mirror
(Hive Bee)
09-11-04 02:33
No 530827
scarmani, excellent and informative post!!!
Nicodem, sorry for my shoddy adaptation of a part of the synthesis for DOAM found in _PiHKAL_ to a totally different compound. I don't really have easy access to many journals where I live now, but all these piperidyl stimulants have really peaked mine, and I hope yours and some other peoples', interest.
In particular, scarmani's mention of phacetoperane (phenyl-2-piperidylmethanol) caught my eye. Although I know I am risking sounding like the village chemistry idiot for not knowing the answer to this simple question already, I'm going to ask it anyway:
Structurally (pseudo)ephedrine is to methamphetamine as phacetoperane is to 2-benzylpiperidine. Namely, the removal of the benzylic hydroxyl group of each of the former compounds leads to the latter drugs.
Phacetoperane, while (mildly) controlled in 1st world countries for being a stimulant, is easily obtainable from overseas; a simple Google search showed me this in a matter of minutes.
Now, here's the big if. Can commercially available phacetoperane hydrochloride be reduced with red phosphorous and iodine to yield 2-benzylpiperidine (2-BzP)?
I don't see why not, but I am no expert (yet). ;)
Nicodem, if not, then what article do you need to have looked up for adaptation to a possible 2-BzP synthesis? I think I could tell from one of your posts, but I want to be sure before trekking over the river and through the woods to the university library and then digging through the ponderous scientific literature for hours on end with no guarantee of finding the article to begin with. Please be specific if you can. Thanks.
Scarmani mentions that phacetoperane faces the same limitations as methylphenidate in that only one of its isomers is active. Well, that is no big deal; just ingest twice as much. Only one of the isomers of methamphetamine is active, but there are seemingly millions of satisfied tweakers nonetheless, right? The other negative thing mentioned was the phacetoperane is weaker than Ritalin. However, upon removal of the hydrophilic -OH group, I have a feeling 2-BzP is going to by VERY active. This is due to the much more lipophilic properties of 2-BzP compared to phacetoperane. Again, the comparison to (pseudo)ephedrine and methamphetamine comes to mind, another blood-brain barrier issue. In that case at the very least, the reduction of the alcohol makes a difference as big as night from day. Only time will tell, with the help of some chemical and pharmacological pioneering, if I'm right about 2-BzP. We are the guinea pigs. Cheers.
The fact that Shulgin first suggested that levophacetoperane might one day become a future drug of abuse only strengthens my resolve to test 2-BzP (which could also be named phenyl-2-piperidylmethane I suppose) for central nervous system activity in humans.
"Get busy, child!"--The Crystal Method
(Hive Bee)
09-11-04 05:08
No 530838
Yes, phacetoperane can be reduced with RB/I method just like ephedrine.
About the activity of 2-benzylpiperidine I know nothing. The reason is that stimulants are not off my interest - that's why I said to check Medline yourself. I don't know exactly what to do with stimulants (except maybe as a study aid).
Anyway, from what I understood phacetoperane is exactly a "desphenyl pipradol" and since pipradol does not act by the same mechanism as (meth)amphetamine I would say that removing the -OH group might not lead to a more potent stimulant. But like I said this is not my area anymore so you should check the difference in activity of pipradol and its deshydroxy analogue. Scarmani should know better on this subject. BTW, that was a great review he prepared. Wish there would be more posts like that one.
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Hive Bee)
09-11-04 08:33
No 530858
Nicodem, I agree with your quote that we need to solve the problem of more and better potential drugs, especially unscheduled ones like 2-piperonylpiperide and 2-benzylpiperidine whose precursors are relatively uncontrolled because they are so obscure.
I'm sorry you don't like stimulants, but I f-ing love them, that's why I'm so excited about stumbling on this seemingly idiot proof synth of 2-benzylpiperidine from commercially available online Chinese phacetoperane hydrochloride, red phosphorous and iodine.
Rhodium, you have always been my hero(ine), will you be the first to synth and bioassay this easily made stimulant?
I am truly not able, for many reasons.
As for you, Nicodem, I'll leave the challenge of coming up with a synth for 2-piperonylpiperidine in between your time studying for finals. You are clearly intelligent enough; in fact, you seem like a real chemistry professor to me. I appreciate your input.
PLUR (Peace Love Unity Respect) and BURP (Bring Us Real Pills),
the cattleprodder (official Hive gadfly)
(Hive Bee)
09-11-04 11:47
No 530897
1st - when I said tomorrow I lied (it`s obvious), excuse me. And one of the results I was going to post was 1-phenyl-3-azabicyclo[3.1.0]hexane. Yes, it`s very similar to DOV 21,947. And it`s analgesic; I don`t know what kind of analgesic (narcotic or NSAID or whatever). Further information will be welcomed (read "please, post something!!!").
cattleprodder - why do you suggest
just ingest twice as much
Just guessing? Let me remind you that there are 2 asymmetric carbon atoms => 4 forms (just like ephedrine, not methamphetamine). But that doesn`t mean that the dose should be 4x, maybe more like 2.342617x.
(Hive Bee)
09-12-04 05:05
No 531003
Phacetoperane is actually the acetate ester of phenyl-2-piperidinemethanol, but the presumption would be that the ester is metabolically removed. I think Nicodem might be right -- 2-benzylpiperidine would probably not be more potent relative to phacetoperane; by analogy to pipradol and related compounds, the hydroxyl group seems to increase potency. However it does seem likely that 2-benzylpiperidine would have activity, although given the simple structure I think it would be mentioned more in the literature if it was anything particularly notable.
As an aside, if anyone is in an adventurous testing mood, 2 and 3 phenyl and benzyl pyrrolidines look directly available without the need for synthesis.
http://forums.lycaeum.org/cgi-bin/ultima
One pipradol-like stimulant subfamily I didn't mention above was:
../rhodium /diarylp
Molecule: 1-methyl-4,4-diphenylpiperidine ("CN1CCC(C2=CC=CC=C2)(C3=CC=CC=C3)CC1")
Also just to note that many substituted 4-phenylpiperidines are scheduled as narcotics (opioids) belonging to the pethedine / prodine family.
boot from the shadow of a broken mirror
(Stranger)
09-12-04 14:02
No 531057
One of the molecules you have drawn is very similar to the structure of Zoloft. That thioindole containing pcp derivative also looked like a fancy heteroaromatic entity. I could see that one being enjoyable.
(Hive Bee)
09-13-04 23:33
No 531237
(Rated as: excellent)
First of all, I’d like to correct a mistake in my previous post (as Scarmani did, but I thought I’d best point it out). The 0.5-1mg dosage referred to
3-(alpha,alpha-diphenylmethyl)morpholine
In addition to the excellent post by Scarmani, there are a few additions to the list of active structures. These are all compounds based on the phenethylamine skeleton, and do not cover the phenyltropane groups etc. Most are definitely active (at least as appetite suppressants), but a few stem from a logical extension of known active compounds, using simple measures such as substituting a sulphur atom for an oxygen atom.
In order to arrange them in groups, I have classified them as follows:
2-Benzylpiperidine derivatives
Phenmetrazine derivatives
Alicyclic and oxygen heterocyclics
Other nitrogen heterocyclics.
Most can be found in assorted journals, but a few come from the work I did for my M.Sc. (SAR of anorectic agents), which was included in a larger paper written by a couple of people who had been lecturers during my undergraduate years (I was eventually relegated on said paper to an et al!).
For the names of the following, I have used the approved drug name for compounds that have had some clinical use (because I’m lazy!). Ones marked with an * are ones that should theoretically have activity, but could not be found by searching (a limited number of) journals.
2-Benzylpiperidine derivatives
1 – 2-benzylpiperidine*
2 – methylphenidate
3 – levophacetoperane
4 – pipradrol
5 – alpha,alpha-diphenyl-2-piperidinemethane
6 - alpha,alpha-diphenyl-2-pyrrolidinemethan
7 - alpha,alpha-diphenyl-2-pyrrolidinemethan
8 - alpha,alpha-diphenyl-3-morpholinemethane
9 – alpha-phenyl-alpha-(2-thienyl)- 2-piperidinemethane*
10- 3-benzylmorpholine*
11- methyl alpha-phenyl-alpha-(3-morpholino)acetate
12- alpha-phenyl-alpha-(3-morpholino)methano
Phenmetrazine derivatives
1 – phenmetrazine
2 – 3,6-dimethyl-2-phenylmorpholine
3 - 3,5-dimethyl-2-phenylmorpholine*
4 – 3-methyl-2-phenylmorpholine-6-one
5 - 3-methyl-2-phenylthiomorpholine*
6 – phenmetramide
7 - 3-methyl-2-(2-thienyl)morpholine*
8 – phendimetrazine
9 – ring substituted phenmetrazine*
Alicyclic and oxygen heterocyclics
1 – fencamfamine
2 – tranylcypromine
3 – 2-phenylcyclopentylamine
4 - 2-phenylcyclohexylamine
5 – 3-amino-2-phenyltetrahydropyran*
6 – 2-aminoindane
7 – 2-aminotetralin
8 – 3-aminochroman*
Other nitrogen heterocyclics
1 – 2-methyl-3-phenylquinuclidine
2 – 2-methyl-3-phenylpiperidine
3 - 2-methyl-3-phenylpyrrolidine*
4 – alpha-(1-pyrrolidyl)propiophenone
5 – prolintane
6 – pyrovalerone
7 – pemoline
8 – aminorex
9 – 4-methylaminorex
As regards the refs., see Scarmani’s comment (but if someone is really interested in one particular compound, I suppose I could make the effort).
If I had to choose one to synth, I would go with one of the morpholine derivative, purely for ease of synthesis. Hope this is of use to someone!
PS - In terms of anorectic activity, compounds that inhibit re-uptake (of dopamine) are far less active than ones stimulating its release (a year of work summed up in a couple of lines - isn't science great!)
That is right, the Mascara Snake: Fast and bulbous
(Hive Bee)
09-14-04 19:49
No 531364
(Rated as: good read)
> One of the results I was going to post was 1-phenyl-3-azabicyclo[3.1.0]hexane. Yes, it`s very similar to DOV 21,947. And it`s analgesic; I don`t know what kind of analgesic (narcotic or NSAID or whatever). Further information will be welcomed
1-Phenyl-3-azabicyclo[3.1.0]hexane derivatives as new ligands for sigma receptors
Agostino Marrazzo, Andrea Pappalardo, Orazio Prezzavento, Franco Vittorio, and Giuseppe Ronsisvalle,
Arkivoc. Vol 2004(5), 156-169
Abstract:
A series of 1-phenyl-3-azabicyclo[3.1.0]hexanes were synthesized as more conformationally restricted prototypical ó ligands 3-phenylpiperidines with the aim to developing new ó ligands. Compared with 3-phenylpiperidines reported by Largent et al., binding data showed that conformational restriction was not detrimental for ó receptor affinity. Specifically, except for secondary amine 4, all racemic 1-phenyl-3-azabicyclo[3.1.0]hexane derivatives (12-19) showed moderate to high affinity for both ó1 and ó2 receptors. Dextrorotatory isomers with the same configuration of 3-phenylpiperidines to C-1 carbon linked to the phenyl ring showed a better affinity and selectivity for ó1 receptors compared to the respective levorotatory isomers. Compounds (+)-14 and (+)-15 displayed very high affinity for ó1 (Ki = 0.9 and 2.3 nM respectively) but low selectivity for receptor subtypes. Compound (+)-18 with N-phenethyl substituent embodies the highest selectivity for ó1 receptors... Sigma (ó )receptors are typical binding sites interacting with several psychoactive drugs including haloperidol, benzomorphans and phencyclidine. The ó1 subtype exhibits high affinity for (+)-benzomorphans such as (+)-pentazocine and (+)-N-allylnormetazocine (SKF-10,047) and a reduced affinity for the respective (–)-enantiomers. Based on animal model studies, this subtype seems to be involved in cocaine induced behavioral changes, in opiate induced analgesia, steroid-induced mental disturbances and alterations in immune functions.
http://www.arkat-usa.org/ark/journal/200
So, it looks like N-substitution of this structure gives sigma ligands (though apparently not the unsusbtituted molecule). This might be an explanation for analgesia. Just as unrelated speculation, sigma affinity could possibly explain the odd effects of http://www.erowid.org/library/books_onli
boot from the shadow of a broken mirror
(Hive Bee)
09-17-04 16:05
No 531859
Fastandbulbous, scarmani, Rhodium or Nicodem,
Is pipradol a stimulant or an analgesic? Edit: It's a stimulant. I'm not suggesting that 2-benzylpiperidine is an analgesic, and thus falls under the SAR umbrella of pipradol as Nicodem suggests, but rather a stimulant under the methamphetamine SAR domain.
Then again, MDMA is both a stimulant and an analgesic, so who knows? SARs are largely conjecture and discussions of them often degenerates into different peoples' pure, theoretical and rather pointless speculation (including my own at times).
In fact, computerized SAR chemistry is one of the biggest failures of modern chemistry as far as I can tell; in fact, when I was still in college they closed the new, multimillion dollar computational chemistry department because it had produced no quality research.
What I'm trying to say is, all this theory is fine, but I just want to actually taste some
2-piperonylpiperidine and 2-benzylpiperidine based on my hunch, which is itself based on cut and paste chemical structure manipulation (the "me too" drug approach in the pharmaceutical world's jargon), that they will have psychoactive properties in common with Ritalin, ecstasy, meth and perhaps be even better.
Until these compounds are made and synthesized and taken either by someone or at least some lab animals, then we will never really know what the acitivity is. Nicodem suggested that I search medline myself, but according to Fastandbulbous, who has apparently studied this field extensively, such a search would be futile (as he put an asterik by the 2-benzylpiperidine entry in his post, meaning that the compound is expected to have activity but does not show up in the scientific literature).
However, I do agree with scarmani's statement that if
2-benzylpiperidine were especially active (say, at least as active as methamphetamine), then we would probably know of it by now, but hey, you never know.
Henry Ford at one time believed that the Model T was such an advanced automobile that it could not be improved on. However, this attitude leaves no room for scientific advancement because it implies that everything in the world has already been discovered and is known. I agree that there is nothing new under the Sun and perhaps the amount of knowledge in the universe is in fact infinite, but if that is true, then more has been forgotton over the aeons than has been remembered.
Also, I would like to thank scarmani for pointing out that phacetoperane hydrochloride is actually the acetate ester of 2-piperidinylphenylmethanol. However, this is only a minor problem because then all one has to do is hydrolyze off the acetate ester with a simple acid or base reflux to get the benzyl alcohol and then reduce the benzylic alcohol with either anhydrous ammonia and lithium or red phosphorous and iodine to yield 2-benzylpiperidine.
Buying phacetoperane HCl from China and synthesizing
2-benzylpiperidine actually looks easier than making MDMA in many respects. From what I could tell from a quick online search, phacetoperane is not a controlled substance in the US but may be mildly controlled in Canada and Europe.
The fact that 2-benzylpiperidine (and its methylenedioxy analogue) are seemingly that unexplored and that easy to make, at least in the case of the former anyway, from commercially available and OTC products makes this project very interesting to me.
That is why I would very much appreciate it, Fastandbulbous, if you could dig up any information you may have or any of your research articles or other information regarding 2-benzylpiperidine or even 2-piperonylpiperidine. If someone knows how they make phacetoperane commercially I would also be grateful if they would share. I am interested in knowing the latter synthesis in hopes that it will elucidate a synthetic outline towards 2-piperonylpiperidine, which is one of my ultimate goals.
Even if I don't get any more answers to these questions, this has been an excellent, informative post, you guys. Thank you for contributing so voluminously. Scarmani's post alone is almost enough to justify archiving this thread in some way, but that would be of course up to Rhodium.
longimanus,
I meant you would have to eat 2x as much racemic
2-benzylpiperidine, which has only one chiral center and therefore only 2 possible stereoisomers, to get the same effect as eating only x amount of the active stereoisomer of 2-benzylpiperidine. (Phacetoperane has, as you pointed out, two chiral centers and thus 4 possible stereoisomers, but I wasn't talking about eating that one.) This crude calculation, as you pointed out, is based on the assumption that one and only one of the stereoisomers retains all psychoactivity and that the other ones have none, an assumption which I am sure is not always the case but seems to be right on target in the case of methamphetamine at least.
scarmani,
I have tried 4-fluoroamphetamine and was not all that impressed. The florine atom, while very electronegative, is not much bigger than a hydrogen one, so one tends to get better pharmacological results with going up a size to at least a chlorine atom for all of one's designer drug needs. (For example, 2CI is stronger per gram than 2CB from what I've heard.) I have a feeling 3,4-difluoroamphetamine would be better than 4-fluoroamphetamine, but I would rather place my bet on 3,4-dichloromethamphetamine, taking an aromatic structural cue from sertraline (Zoloft), which also has a 3,4-dichlorobenzene ring. The most selective of all the SSRIs, however, is paroxetine (Paxil) and it has the coveted 3,4-methylenedioxybenzene ring system. Sesamol, the natural product chemical, is actually used to make Paxil I think.
As for 3-methoxy-4-ethoxy(meth)amphetamine, which can definitely be made starting from diethylsulfate and cheap, plentiful, unwatched oil of eugenol, I say GO FOR IT! I am simplying itching for a real live bee to try this one. According to Barium, the PEA version of this compound is pleasant and mood elevating. I still can't figure out why Shulgin never tried 3,4-DMA either, but I guess he had his hands full.
Finally, Nicodem, you mention 3-phenylpyrrolidine. I know you don't like or at least don't know what to do with stimulants, but do you like tobacco products, because N-methyl-2-(3-pyridinyl)pyrrolidine is none other than nicotine itself?
How 'bout N-methyl-2-phenylpyrrolidine? Scarmani intimated a little while ago that these are already commercially available. Who wants to be the first crackhead to smoke some? Why did I select N-methyl-2-phenylpyrrolidine?
I know that pyridine is less aromatic when compared to benzene because the lone pair of electrons on the heterocyclic nitrogen are at right angles to the conjugated pi system of the larger ring as a whole.
Perhaps synthesizing nicotine with a benzene ring instead of a pyridine ring would result in a better (read, more addictive) product or perhaps it would be not fit any of the same receptors anymore without the lost nitrogen and be inactive. At any rate, I'm going to shut up for now and go smoke a good ole cigarrette and then munch on some more phendimetrazine.
As Shulgin wrote somewhere in _PiHKAL_, "and visions of sugarplums danced in their heads."
MURP (make us real pills)
(Hive Bee)
09-18-04 02:17
No 531960
Also, I would like to thank scarmani for pointing out that phacetoperane hydrochloride is actually the acetate ester of 2-piperidinylphenylmethanol. However, this is only a minor problem because then all one has to do is hydrolyze off the acetate ester with a simple acid or base reflux to get the benzyl alcohol and then reduce the benzylic alcohol with either anhydrous ammonia and lithium or red phosphorous and iodine to yield 2-benzylpiperidine.
If you ever want to use the rP/I method to reduce phacetoperane than I strongly suggest you to leave that acetate ester as it is. Acetates are reduced even faster since the acetoxy group is a much faster leaving group than the hydroxy.
Finally, Nicodem, you mention 3-phenylpyrrolidine. I know you don't like or at least don't know what to do with stimulants, but do you like tobacco products, because N-methyl-2-(3-pyridinyl)pyrrolidine is none other than nicotine itself?
Nicotine acts at the nicotinic receptors and the pyridine nitrogen is essential in this interaction. Your reasoning makes no sense in neurochemical context. It is even much more far out than, for example, claiming MDMA is psychoactive because it resembles amphetamine. But the contribution of MDMA in dopamine/noradrenaline release is much lower than its contribution in releasing serotonin and inhibiting its uptake. And still here we talk about related neuromodulatory systems while the acetylcholine system is something completely unrelated and is actually a real neurotransmitter system (that is, not just neuromodulatory). If such reasoning would still be actual you would have people claiming that DXM is active because it resembles morphine and other opiates even if it is of the opposite stereochemistry. Yet the interaction of DXM with opiate receptors is negligible.
The only thing that applies here is the key into the lock example. You have to have a key that can accommodate in the lock and certain points on the key that will turn the lock into the wanted position and open or close the door/receptor. It is also important that this key/molecule can pass all the obstacles to come into the contact with the lock/receptor site.
PS: Nicotine is 3-(N-methyl-2-pyrrolidinyl)pyridine and its structure is not much related to the phenyl ring substituted 3-phenylpyrrolidines that I proposed.
“The real drug-problem is that we need more and better drugs.” – J. Ott
(Hive Bee)
09-18-04 06:29
No 531979
Nicodem,
Good to hear from you again! I apologize for misnaming nicotine. Now let's discuss drug discovery and logic and examine how we think differently about science and drug discovery and what, if anything, that means.
First of all, thank you very much for your advice not to hydrolyze the acetate ester before reducing with red phosphorous and iodine or anhydrous ammonia and lithium. It is this kind of practical advice that is invaluable to all chemists, and theoretically, the advice also makes sense based on the reaction mechanism and the fact that an acetate ion is a much better leaving group than a hydroxyl moiety.
Apparently, the total synthesis of 2-benzylpiperidine gets easier every day. A few days ago we were looking at starting from scratch with benzene, and now the whole scheme seems workable in one step from OTC and internet ordered chems (not that I'm planning anything like that because I'm not).
Second, you are right in that the 3-phenylpyrrolidine that you mention has little to do with nicotine. If nicotine did not require its pyridine ring in order to maintain its psychoactivity, then my mention of 2-phenylpyrrolidine would have possibly seemed less off the wall from your perspective, or maybe my thinking invariably seems weird when compared with yours--not necessarily a good or bad thing, mind you, just a fact to be noted.
However, now let's debate chemical theory as it relates to drug discovery. Of course, no matter how one goes about the process, each drug must be isolated and tested for desirable and undesirable side effects. However, there seem to be two main schools of thought with respect to drug discovery and science and religion too for that matter: inductive thinkers (who take small bits of information from varied sources and then extrapolate their findings to the big picture) and deductive thinkers (who are the typical rigid scientist type who is detail oriented and does not believe in making connections between and among academic disciplines). The scientific community prizes detailed, deductive, specific, specialized information and does not try to find patterns in the big picture. I, however, am an unconventional, inductive thinker and proud of it; I agree with Shulgin when he says that chemistry is an art, and I have more of an artist's temperament than that of a researcher. Not remarkably, I have had, in the past, trouble in academic and professional settings. For example, I believe in mythology, astrology, Hinduism, Christianity and science and have no qualms linking astrology to sp2 orbitals if necesssary.
Anyway, here is an example of my "cut and paste the chemical structure approach to drug discovery and understanding." I fully expect conventional thinking scientists like Nicodem to disdain this approach but it sometimes nevertheless holds true. However, this method was somewhat introduced by Shulgin in the guise of the "try it and see" approach.
It is obvious, as Nicodem has already pointed out, that DXM bears obvious but only distantly related structural resemblence to the poppy based opiates, such as codeine, morphine, hydrocodone and oxycodone. As an inductive generalist, I therefore would not be overly surprised if dextromethorphan acts on at least some of the known opiate receptors, but probably not a lot of them. Low and behold, if I recall correctly, DXM acts on the mu (or was it sigma?) opiate receptor to produce its cough suppressing and ketamine like disassociative states, although being only distantly related to the traditional opiates, DXM is not surprisingly a poor pain killer.
Another synthetic opiate, pentazocine is known to act on the mu or was it sigma? opiate receptor and also produces hallucinations at high doses, yet pentazocine is a much more effective painkiller than DXM (probably not when compared to levorphanol, however).
One point that I'm getting at is that I agree wholeheartedely with the Transformer I had as a child whose motto as printed on the toy's box said, "Molecular structure is the key to understanding." I love organic chemistry; it is the only symbolic form of reasoning that connects the tiny to the infinite and can be taylor made to physically create a novel molecule from a preconceived diagram. I feel the organic chemistry should be taught in high school. In fact, I feel sorry for people who can't read Kekule chemical drawings. However, I realize that I am the exception in the modern world of science in that I generalize and inductively reason to the point of connecting astrology to sp2 orbitals. Also, as a right brained thinker, I can't prove a lot of my ideas and I'm not good at coming up for the reasons I thought of something, but the years of being flamed have paid off, and now I'm proud to be different from the herd. I realize distinguishing between genius and idiocy can at times be difficult, but bear with me.
Tinkering with the chemical structure methodology has long been used in the pharmaceutical industry. It works something like this. One company comes up with a good drug, usually by trial and error, accident or plain good luck. Next, 20 other companies furiously tinker with the structure until they find a "me too" drug that they can then patent. This is how we end up with 30 different kinds of benzodiazepines, and that, in my opinion, is a good thing. I don't know what the first benzodiazepine to be invented was or how it was discovered, but I feel quite sure a computer did not predict jack shit about it. This is also the process that, starting with penicillin, 2nd, 3rd and 4th generation antibiotics came about. If this theory of drug discovery is applied to Shulgin's life work then _PiHKAL_ would be a compendium of "me too" drugs based on dopamine (DA), while _TiKHAL_ would be a compendium of drug information based on molecules of the "me too" type of serotonin (5-HT). DA and 5-HT are arguably the most important neurotransmitters involved with consciousness, so it is not surprising to me that tinkering with them yields interesting results.
Shulgin, however, is or at least was of the opinion that the mimickry aspect was imaginary or at least unprovable. However, after experiencing MDMA (a DA mimicker), TMA-2 (a 6-OH-DA mimicker) and TMA-6 (which has in common with DA only the 4-MeO functional group), I can verify that MDMA is the most pleasurable and the other two are marginal at best. It is on this mimickry aspect that Shulgin's views diverge from mine; this is probably why he never chose to taste 3,4-DMA, while I would predict it to be Ecstasy like. In the case of psilocin the similarity between it and 5-HT is undeniable. Another example is GHB and GABA.
For example, according to my theory, I fully believe the reason that MDMA gets you high is that it is shaped like a dopamine molecule made just lipophilic enough to cross the BB barrier and that MDA makes you more likely to hallucinate because DA is implicated in schizophrenia MDA resemembles DA more closely than does MDMA.
Years ago, when perusing my 1996 _Physician's Desk Reference_, I made an interesting observation: almost all the psychotropic drugs (at least 50 to 60%) had a phenylethylamine skeleton embedded in their structure somewhere or another. When I look at 2-benzylpiperidine, I see a cross between methamphetamine and methylphenidate (Ritalin) but longer lasting due to the body's relative inability to deaminate the drug. When I look at the symbol for 2-piperonylpiperidine, I see Ecstasy crossed again with Ritalin. When I look at morphine and then at MDxx, I see a common
3,4-dioxyphenylethylamino moiety and I realize that narcotic pain pills at times feel a lot like rolling. I also realize that methamphetamine and MDMA wake you up in a similar fashion and sharing the amphetamine scaffolding. I could go on (for example, I am willing to wager that you would be amazed at what happens when adding a 3,4-methylenedioxy unit to the phenethylamine portion of fentanyl), for the list is endless (for example, shrooms are just N,N-dimethylated serotonin with the hydroxyl group in a slightly different location), and of course my theory is not always right, but the future of drug abuse may depend on the art of mimickry more than anything else. Again, to quote Shulgin, psychopharmacology depends on a number of variables such as intuition, chemical skills and luck but not necessarily logic.
That, I think, is why I love it so. NOW WOULD SOMEBODY COOK UP SOME 2-benzylpiperidine, 2-piperonylpiperidine, 3-methoxy-4-ethoxy(meth)amphetamine and 3,4-dichloromethamphetamine. Thanks.
(Hive Bee)
09-20-04 18:44
No 532413
It is obvious, as Nicodem has already pointed out, that DXM bears obvious but only distantly related structural resemblence to the poppy based opiates
Cattleprodder, although DXM does not show opiate like activity, laevomethorphan and laevorphan are active as classic opiates, the former being equated with codeine and the latter with morphine; in fact, so much so that in the UK they have been listed in the Misuse of Drugs Act. It is merely that the conformation of DXM does not allow it to interact with the mu receptor (I do believe that DXM acts via the one of the sigma receptors).
A couple of other things: I'm sure that 4-chloroamphetamine is quite neurotoxic to serotonogic neurons as are 4-bromo and 4-iodoamphetamine, so 3,4-dichloroamphetamine may well be neurotoxic as well (but I have no data to back that statement up, just a hunch)
Last of all; for all of the dopaminergic drugs that act by stimulaing release of dopamine (as opposed to re-uptake inhibitors), there is a direct correlation between anorectic activity and the drug's CNS stimulant activity, so the better the appetite supressing properties, the higher the abuse potential. The anorectic correlation does not carry over to anorectics that exert their action on serotonogic systems (such as fenfluramine and sibutramine), although when E. Merck first synthed MDMA beck in 1912, they were looking at it for it's appetite supressing activity
That is right, the Mascara Snake: Fast and bulbous
(Hive Bee)
09-21-04 01:32
No 532461
Fastandbulbous,
For some reason, all the 3-only-substituted (3-MeO-amphetamine, Wellbutrin / N-tert-butyl-3-chlorophenylcathinone) or 3,4-disubstituted amphetamines of which I know (MDMA, MDA, MDE, methylone--which I know is strictly speaking a disubstituted methcathinone, and
3,4-DMA) are all very much less toxic than the 4-only-substituted amphetamines (4-MTA, 4-PMA,
4-methylmethamphetamine, and 4-PMMA), perhaps due to MAO inhibition of the latter category combined with stimulating properties as well. Also, the larger the halogen that is chosen, the more toxic and acute the drug's effects will in general be.
The order of size in the halogen family is that fluorine is smaller than chlorine is smaller than bromine is smaller than iodine. Although fluorine is the most electronegative atom in the whole periodic table, its size--comparable to that of a hydrogen atom--severely limits its psychoactive potential. For example,
4-fluoroamphetamine is very gentle and did not feel neurotoxic to me at all, but it was also weaker than Adderall.
Cl2 is a green gas, but still, I am no more afraid to eat 3,4-dichloroamphetamine than to pop a Zoloft (sertraline HCl), which also shares a 3,4-dichlorobenzene ring to do help it do its dirty work.
On the other hand, 3,4-dibromoamphetamine I would most likely politely decline if offered any. Liquid bromine vapors look vile to me and the color is like that of liquified dried blood. 3,4-difluoroamphetamine I will most likely gratefully try should the opportunity present itself, but I only expect it to be slightly better than 4-fluoroamphetamine.
But, back to the topic of this thread, I have all but given up on synthesizing 2-benzylpiperidine from phacetoperane or 2-piperonylpiperidine from Rimiterol due to the severe lack of availability of these precursor chemicals, even from China.
In some countries phacetoperane (the reverse ester of Ritalin/methylphenidate) is outlawed, in others it is prescribed and in the US it is neither outlawed nor prescribed but still hard to find and also conceivably illegal under the Analogue Drug Act as an isomer of Ritalin, a Schedule II drug. Phacetoperane's dosage (5 to 20 mg per day per person), side effects and indications for use are very similar to Ritalin's.
Pipradol is a Schedule IV drug in the US and therefore must be weak as water. Rimiterol is a bronchodilator in the UK and as such should not have any desirable, abusable properties to speak of. However, it can be transformed into 2-piperonylpiperidine with enough effort. However, doing that would probably not be a very cost effective route either and would be quite laborious.
The lack of availability of these two phenylmethylpiperidines (PMPs) has made me basically declare this project DOA. I give up, which is a shame, but I doubt that either of them would have bettered either MDMA or methamphetamine, which are both based on natural products as their chief precursors, in the drug desirability department and certainly not in the ease of synthesis competition either.
C'est la vie.
(Hive Bee)
09-21-04 04:00
No 532479
(empty)
(Hive Bee)
09-21-04 07:47
No 532500
I have a feeling 3,4-difluoroamphetamine would be better than 4-fluoroamphetamine
It isn't. It is significantly less euphoric than the 4-FA and has a horrible comedown (well, not actually that bad, but horrible given that the effects of the compound are rubbish in the first place). All in all a completely crap drug.
I am no more afraid to eat 3,4-dichloroamphetamine than to pop a Zoloft
Then you must be a fool. One has vastly more clinical data available in humans. You could fry yourself.
Liquid bromine vapors look vile to me and the color is like that of liquified dried blood
That has fuck all to do with neurotoxicity. You ramble.
4-fluoroamphetamine is very gentle
not at 250mg of sulfate salt it isn't/
(Newbee)
09-21-04 21:49
No 532633
regarding these hetereocyclic compounds; does anyone know if a saturated secondary ring is necessary for activity? For instance, is there a possibility that a simple and easily synthesized compound such as alpha-(aminomethyl)-naphthalene would be centrally active?
It appears just about everything with this ethylamine modality protruding from an unsubstituted phenyl ring exhibits some type of stimulatory affect; while ring substitutions may produce anything from analgesics and disassociatives to psychedelics.
- phenethylman -